![[NASA Logo]](../Images/nasaball.gif)
![[NASA Logo]](../Images/nasaball.gif) |
NASA Procedures and Guidelines |
This Document is Obsolete and Is No Longer Used.
|
|
P.1 Purpose
P.2 Applicability
P.3 Authority
P.4 References
P.5 Cancellation
2.1 General Program Support
2.2 Health Promotion and Wellness Programs
2.3 Quality Assurance Program
2.4 Records Management
3.1 Clinical Services
3.2 Physical Examinations
3.3 Emergency Services
3.4 Infection Control Program
4.1 Regulatory Compliance
4.2 Occupational Exposure Assessment and Management
4.3 Occupational Exposure Limits
4.4 Sampling and Analytical Methods and Equipment Calibration
4.5 Ergonomics
4.6 Radiological Health
4.7 Environmental Sanitation
4.8 Balancing Work-Rest Cycles
4.9 Hearing Conservation
4.10 Food Safety
5.1 Key Elements of the Employee Assistance Program
5.2 Workplace Violence
5.3 Domestic Violence
6.1 Management of Workers' Compensation Injuries and
Illnesses
6.2 Interagency Reporting
7.1 Introduction
7.2 Purpose
7.3 Process Description
7.4 Responsibilities
7.5 References
This NASA Procedural Requirements (NPR) describes processes for implementing and managing the NASA Occupational Health Program (OHP). The OHP is the overreaching Agency function encompassing two major components (Occupational Medicine and Environmental Health) and integrating other essential contributors to employee health. This NPR explains how services and reporting procedures for occupational medicine, occupational health nursing, environmental health, and employee support elements are standardized, when warranted and practicable, across the Agency.
This NPG is applicable to NASA Headquarters, NASA Centers and Component Facilities as well as to the Jet Propulsion Laboratory (JPL)and NASA contractors to the extent specified in their contracts.
The authority for this NPG is as follows:
a. 42 U.S.C. 2473(c)(1) of the National Aeronautics and Space Act of 1958, as amended.
b. U.S.C. 668, Section 19,of the Occupational Safety and Health Act of 1970, as amended.
c. Executive Order 12196, dated February 26, 1980, "Occupational Safety and Health Programs for Federal Employees," (3 Code of Federal Regulations (CFR) 1980 Compilation).
d. Basic Program Elements for Federal Employee Occupational Safety and Health Programs and Related Matters, 29 C.F.R. ºº 1960.1 - 1960.90.
a. NPD 1800.2, NASA Occupational Health Program, January 16, 2001
b. NPD 1810.2, NASA Occupational Medicine Program, January 16, 2001
c. NPD 1820.1, NASA Environmental Health Program, January 16, 2001
d. NPD 1830.1, NASA Employee Assistance Program, January 16, 2001
e. NPD 1840.1, NASA Workers' Compensation Program, February 23, 2001
NPR 1800.1, NASA Occupational Health Program Procedures, dated October 16, 2002.
NPR 1820.1, Hearing Conservation, dated March 2, 2001
1.1 This NASA Occupational Health Program (OHP) Guideline was prepared to assist OHP professionals and allied professionals throughout the Agency in their daily tasks to assure the health of employees and a safe work environment. The scope and quality of services provided by OHP personnel at NASA Centers are instrumental in achieving the goal of maintaining optimal health and well-being of the Agency's workforce.
1.2 The elements of the OHP are Occupational Medicine, Environmental Health, Radiological Health or Health Physics, and programs for Health Promotion and Wellness, Workers' Compensation and Employee Assistance.
1.3 The format for the NPR focuses on essential processes and includes descriptions, responsibilities, and flowcharts that illustrate how processes are implemented. Additional reference materials are cited, when applicable. As an NPR, it is distributed and approved through NODIS.
1.4 The NASA Headquarters Office of the Chief Health and Medical Officer (OCHMO) issues policy directives for program activities through NPD's, advocates for program support, provides program resources, and monitors the progress of NPD's and NPR's during the NODIS review process.
1.5 The OHP Office is responsible for the accuracy of the content of the handbook and welcomes critical comments for improvements or suggestions for additions.
1.6 Contact the NASA OHP through the OHP Web site at http://ohp.nasa.gov, the NASA Occupational Health Program Manager, or each center's Medical Director
2.1.1 Technical Support Services
2.1.1.1 Introduction
a. NASA stresses the importance of promoting and maintaining physical and mental well-being of employees, to implement all program components in the best possible manner and to ensure compliance with regulatory requirements. The OHP seeks to preserve and maintain the Agency's invaluable human resources and assures the goal of allowing every employee the opportunity to work in a place free from recognized health hazards.
b. As an aid to reaching this goal, one of the responsibilities of the OHP as stated in NPD 1800.2, NASA Occupational Health Program, is to provide technical support to the OHP personnel at NASA Centers. Support may be given in a variety of forms, including but not limited to the following:
(1) Maintain a current, central registry of all applicable legal and regulatory requirements.
(2) Provide professional consultations for civil service and contractor occupational health personnel.
(3) Provide and/or procure technical reference materials.
(4) Provide and facilitate avenues of communication for the OHP community to share ideas and information.
(5) Collect and disseminate state-of-the-art information.
2.1.1.2 Responsibilities
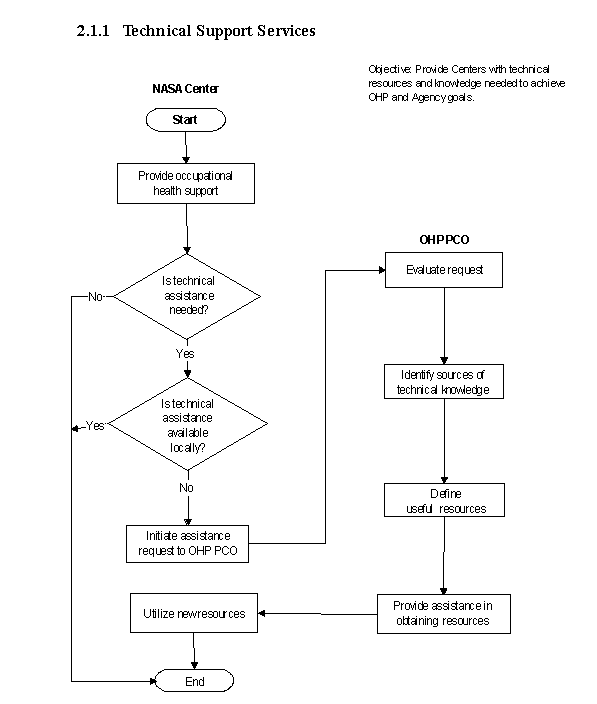
a. NASA Center personnel are responsible for assessing needs to determine if assistance from the OHP is necessary and submitting requests for support.
b. NASA OHP personnel are responsible for providing technical expertise in OHP disciplines and identifying the need for specific technical support. They also provide audits and additional information necessary to evaluate requests that assist Centers in meeting program goals.
c. Reserved.
2.1.1.3 Process Description
a. Reserved.
b. NASA Center-Initiated Request
(1) Identify the need for specific technical support.
(2) Contact OHP and request technical support.
(3) OHP evaluates request and determines course of action.
(4) Provide additional information if necessary.
(5) Reserved.
(6) Reserved.
c. OHP-Initiated Support
(1) Identify specific technical support need through communication with NASA Centers during the audit process, program evaluation, working group meetings, or other contact.
(2) Perform evaluation to determine if specific support is an Agencywide or Center-specific need and gather additional data from NASA Centers as necessary.
(3) Develop plans for the procurement (if necessary), dissemination, and distribution of specific support to the NASA Centers. The plans also include provisions to confirm receipt and utilization by the NASA Centers and, if possible, appropriate and meaningful metrics associated with implementation and use.
2.1.1.4 Flow Diagram
The flow diagram for this process is shown in Figure 2.1.1 at the end of this section.
2.1.2 Reserved.
2.1.2.1 Reserved.
2.1.2.2 Reserved.
a. Reserved.
b. Reserved.
2.1.2.3 Reserved.
a. Reserved.
b. Reserved.
c. Reserved.
d. Reserved.
(1) Reserved
(2) Reserved
(3) Reserved
(4) Reserved
(5) Reserved
(6) Reserved
(7) Reserved
(8) Reserved
2.1.2.4 Best Practices
a. The OHP researches and obtains internal and/or external best practices to complement or augment services provided by OHP personnel at the NASA Centers.
b. Implementation
(1) Discrete mechanisms which aid implementation are as follows:
(a) OHP Web-site capability, allowing e-mail to be sent to all Agency OHP personnel.
(b) OHP Web-site provision of training on discipline specific topics and links to other sites for training and education.
(c) Occupational Health e-mail discussion group.
(d) Distribution lists for e-mail that display pertinent information dealing with work experience information.
(e) Teleconferencing.
(f) Video Teleconferencing System (ViTS).
(g)Periodic conferences and meetings.
(2) Reserved.
(3) Reserved.
2.1.2.5 Flow Diagram
The flow diagram for this process is shown in Figure 2.1.2 at the end of this section.

2.1.3 Education and Training of Occupational Health Professionals
2.1.3.1. Introduction
a. To meet its goals and fulfill its mission, the NASA OHP must have and retain knowledgeable and competent professionals. This requires that NASA Center OHP personnel possess basic knowledge, credentials, licensing and skills relevant to their designated health professional position. These OHP professionals have an obligation to keep up to date on advances in their field. In addition, NASA policy states that its health professional teams must pursue excellence by maintaining awareness of the most current knowledge and processes related to all disciplines required at NASA Centers. For these reasons OHP professionals must have access to all the training needed to reach their full potential.
b. It is also required that all current professional licensures, certifications, and accreditations necessary for professional operations be maintained. Maintaining required licensure and certifications dictates certain continuing education requirements for health professionals.
2.1.3.2 Responsibilities
a. NASA Center Directors are responsible for implementing and operating occupational health programs in full compliance with NPD 1800.2, NASA Occupational Health Program. This includes establishing effective organizations to fulfill occupational health requirements using professionally qualified persons and allocating resources for the HP.
b. Reserved.
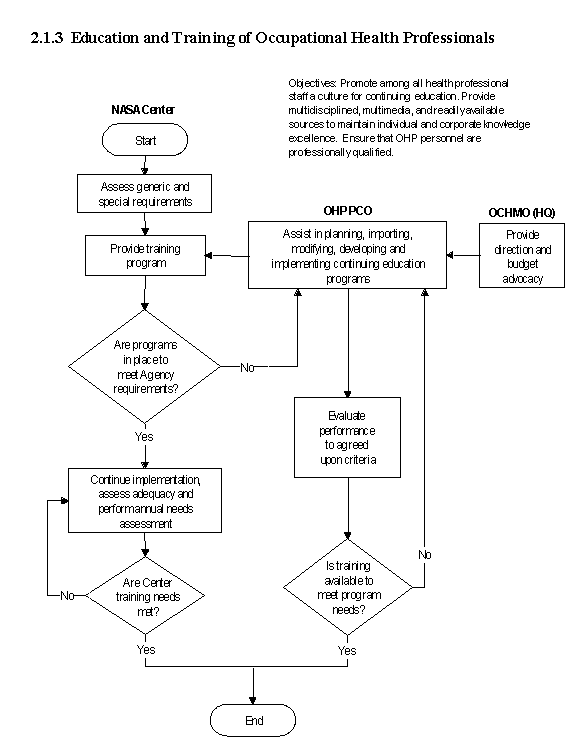
c. The OHP works with NASA Headquarters and the NASA Centers to determine the most appropriate education and training opportunities to be provided each year.
2.1.3.3 Process Description
a. NASA installation hiring policies must comply with Federal and NASA requirements for professional education standards and where applicable, with State licensure statutes.
b. NASA Center OHP managers conduct assessments of professional and career development needs to determine the training needs of their staff.These needs are communicated to Center management and the OHP. Maximum use of telecommunication technologies assures the most efficient educational opportunities and resources.
c. Reserved.
(1) Reserved
(2) Reserved
(3) Reserved
(4) Reserved
(5) Reserved
d. To implement NASA policy in professional education and training, the OHP encourages and contributes to several forms of in-house training programs.These include, but are not limited to the following:
(1) Assuring availability of the most current and authoritative written reference materials.
(2) Providing ready access to the Internet (and specifically to the National Library of Medicine's search sources), the OHP Web site, and to intercenter and external consultants.
(3) Providing regular live and taped audio and video training courses.
(4) Conducting an annual Occupational Health Conference covering all OHP disciplines.
(5) Promoting intercenter and external events (courses, workshops, seminars) on essential topics by discipline experts.
(6) Conducting hands-on training in procedures and simulation exercises to refresh and augment skills where handling of actual events is infrequent.
Employees are able to receive specialized training in discipline-specific topics, as well as earn continuing education credits in occupational medicine, occupational health nursing, environmental health, and employee support programs. The key in-house training opportunity for health professionals is the NASA Occupational Health Conference. Discipline- specific conferences, such as those for environmental health and employee assistance programs, are also provided depending on employee availability and needs documented throughout the year. Satellite downlink broadcasts are also provided to employees when relevant topics become available.
e. Key steps the OHP uses in designing, implementing, and modifying a training and career development program are as follows:
(1) Perform a needs assessment for the institution.
(2) Customize the needs to the institution commensurate with budgetary limitations and available training time.
(3) Establish clear training and career development objectives.
(4) Establish clear performance criteria.
(5) Reserved.
(6) Reserved.
(7) Make modifications to the training program based on the results.
(8) Measure performance to goals.
f. Feedback to the OHP on availability and efficacy of current, and on anticipated benefit from planned, educational programs is routinely sought. From identified Center needs, the OHP determines which opportunities shall be offered, both new and repeated content.
2.1.3.4 Reference
NASA OHP Web site athttp://ohp.nasa.gov/topics/training
2.1.3.5 Flow Diagram
The flow diagram for this process is shown in Figure 2.1.3 at the end of this section.
2.1.4 Web Site Initiatives and Capabilities
2.1.4.1 Introduction
a. The NASA OHP Web site, located at http://ohp.nasa.gov, is the primary resource for quick dissemination of information to NASA OHP personnel. It assists in meeting the primary responsibilities of the OHP to NASA Centers.It serves as a repository of information for OHP personnel to utilize in performing their jobs. It also serves the NASA workforce, both civil service and contractor, and the general public.
b. The OHP Web site provides ongoing technical support to the NASA Centers, such as the following:
(1) Occupational Health Program Directories.
(2) Policies and Standards.
(3) Occupational Health Disciplines (Employee Assistance, Environmental Health, Occupational Medicine, Physical Fitness, and Workers' Compensation).
(4) Occupational Health Topics.
(5) Employee Health Information.
(6) Training Programs.
(7) Occupational Health Program Handbook Desktop Resources.
(8) Resources.
2.1.4.2 Responsibilities
a. OHP personnel at NASA Headquarters and NASA Centers evaluate the contents and make recommendations for changes when necessary.
b. The OHP is responsible for managing the OHP Web site and ensuring that current information is available for use by all OHP personnel.
2.1.4.3. Process Description
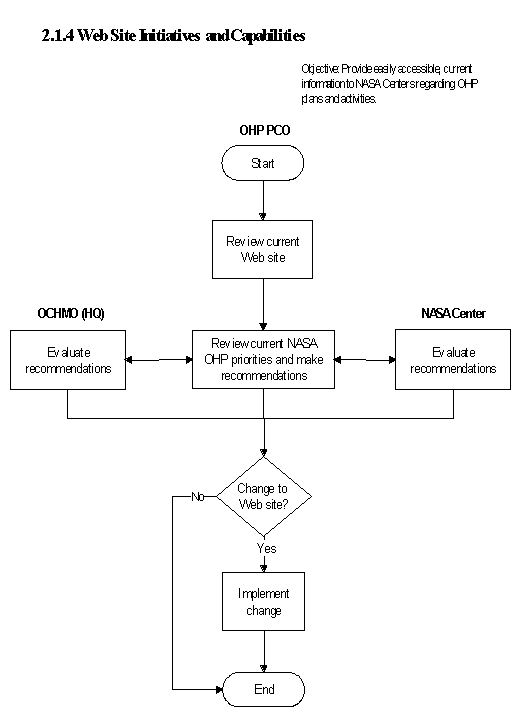
The NASA OHP Web site is an ongoing process of the NASA OHP. The goal is to increase employee knowledge and facilitate communication among the NASA OHP personnel. Specific actions for achieving this goal are as follows:
a. Review current Web site.
b. Review current NASA OHP priorities with NASA Headquarters and all NASA Centers.
c. Request changes, if necessary, to the NASA OHP Web site.
d. Regularly update the OHP Web site.
2.1.4.4 References
The OHP Desktop Resources, a new addition to the OHP Web site, is a dynamic element which provides inter-Center communications, additional up-to-date reference materials supporting topics in the handbook, new or revised drafts of documents in the review process for comments and suggestions, general information on topics of interest to the Centers, and formats and forms for obtaining audit and other types of data required for Agency reports.
2.1.4.5 Flow Diagram
The flow diagram for this process is shown in Figure 2.1.4 at the end of this section.
2.2.1 Fitness Centers
2.2.1.1 Introduction
a. As seen in the publication of the 1996 Report of the U.S. Surgeon General on physical activity and health, physical activity is at the top of our national public health agenda. Today, nearly one fourth of adult Americans have some form of cardiovascular disease. One of the major risk factors of cardiovascular disease is physical inactivity. The objective is to reduce cardiovascular disease by promoting regular exercise. To encourage physical activity in employees, on-site fitness facilities should be established and maintained, or membership in a local fitness center should be offered to employees. The fitness center should consist of shower facilities, an exercise room for stretching and classes, a walking/jogging trail, and a variety of indoor equipment such as treadmills,stair climbers, strength training machines, and free weights. Fitness machines should be equipped or devices should be readily available to monitor heart rate and blood pressure while exercising.
b.One of the OHP goals is to minimize sick absences and reduced productivity due to marginal physical disability, permanent disability, or premature death. This shall be accomplished by providing to employees physical fitness programs to help control and reduce health risk factors, such as sedentary lifestyle, obesity, high blood pressure, and diabetes.
2.2.1.2 Responsibilities
a. The fitness center personnel involved in management or delivery of exercise programs are responsible for obtaining formal training and must have required experience as established by the American College of Sports Medicine (ACSM) to ensure that clients are provided with safe, effective programs and services. Personnel should include the manager, fitness director, and fitness professionals. All personnel shall maintain Cardiopulmonary Resuscitation (CPR)/ Basic Life Support (BLS) certification and be trained in the Bloodborne Pathogen standard
b. The manager is responsible for the overall management of the facility and should be proficient in business, as well as design and delivery of exercise programs. This function is also responsible for fitness environment safety, emergency procedures, and ensuring that members and staff have received a health screening.
c. The fitness director function is responsible for program design, ensuring training, and staff supervision and accreditation, while managing the facility's exercise and activity programs. A degree in exercise science or another health-related field with at least 1 year of supervisory experience in the fitness industry is required. This position also requires professional certification at an advanced level by a nationally recognized health/fitness organization comparable to the ACSM health fitness instructor certification.
d. The fitness professional provides instruction to individuals in exercise and behavioral skills and should have a degree in exercise science. A professional certification from a nationally recognized health/fitness organization (comparable to ACSM exercise leader certification) is also preferred.
e.Facilities providing services in allied health fields such as nutrition or physical therapy should be certified, licensed, or registered with the State as required by law. In order to keep up to date with the most current information, all degreed professionals should attend professional development courses or conferences.
2.2.1.3 Process Description
a. The ACSM and the American Heart Association (AHA) recommends that all facilities offering exercise equipment or services should conduct cardiovascular screening of allnew members. The Physical Activity Readiness Questionnaire and the questionnaire developed by the Wisconsin Affiliate of the AHA are examples of screening instruments. The purpose of doing screenings prior to using the fitness facility is to identify individuals at risk for a cardiovascular incident while exercising. In addition to the questionnaire, blood pressure should also be evaluated. If any question suggests a potential medical problem such as recent or chronic illness or injury, or the blood pressure is 140/90 or higher, the individual shall be referred to either the Occupational Health clinic or their Private Medical Doctor (PMD) for counseling.>Regardless,and a written medical clearance prior to their use of the facility is required. Continued use of the fitness facility is established through renewal of the clearance process on a periodic basis. The results of the information should be kept on file and positively identifiable with the facility user.
b. For safety reasons unsupervised fitness facilities should not operate. It is recommended that all staff be well qualified and appropriately trained. If there are any unsupervised facilities not under contract or sponsored by NASA Centers, users of the facility must have identification on their person and signs must be displayed prominently, advocating a buddy system, and prompting users about medical problems such as chest pain, dizziness, bone or joint problems, and advising users with any of these symptoms to seek doctor's advice. The design of such a facility shall meet ACSM standards for an unsupervised facility. Use of a facility by any employee without someone else available to assist shall be discontinued.
c. A method should be enforced to identify (badge, keyed lock) members who have been screened and eligible to utilize the facility. Individuals are required to at least sign in each time they use the fitness center, or ideally through a computerized system in which statistical information could be extracted to monitor improvement and use. The hours of operation should be able to meet the majority of employees' needs and work schedules. Supervisors should encourage and support participation and the use of flextime work schedules in order to accommodate use of the fitness facility by employees throughout the day. Health observances such as the Health and Safety Day and National Employee Health and Fitness Day should be incorporated into the fitness center and health promotion activities at each Center. A mechanism for customer feedback should also be in place such as an annual survey for continued quality improvement.
d. All fitness facilities must have written emergency policies and procedures. A physician, registered nurse, or emergency medical technician trained in advanced cardiac life support is recommended to be a medical liaison responsible for critiquing emergency drills and reviewing medical emergency plans and incident reports. All fitness center staff should be certified in CPR and first aid prior to employment. A first aid kit containing bandages, gloves and a pocket mask shall be available to the staff for emergency use. Automatic External Defibrillator (AED) availability and training shall meet any Federal or State requirements. Staff training shall be done as soon as possible after employment with emergency drills practiced once every three months. Transportation of employees to a hospital and telephone access to 911 or the local emergency medical system identified on the telephone must be addressed in the emergency plan. A hard-wired system with a large visible emergency button to call medical, first aid, and security and prominently displayed sign is strongly encouraged. A staff member should remain with the employee at all times. Emergency policies and procedures that are regularly reviewed and practiced by the fitness center staff will optimize prompt emergency care to the employee.
2.2.1.4 References
a.NPD 1810.2A, NASA Occupational Medicine Program.
b. AHA/ACSM Scientific Statement, "Recommendations for Cardiovascular Screening, Staffing, and Emergency Policies at Health/Fitness Facilities." 1998.
2.2.1.5 Flow Diagram
The flow diagram for this process is shown in Figure 2.2.1 at the end of this section.

2.2.2 Health and Wellness Programs
2.2.2.1 Introduction
NASA Center health and wellness programs, including extensive stress management programs support the Agency's commitment to the health of its workforce. Health and wellness programs are designed on three levels. These levels include awareness and education, life-style behavioral change, and a supportive environment.
2.2.2.2 Responsibilities
a. NASA Center OHP personnel are responsible for assessing, planning, implementing and evaluating health promotion programs based on the needs of the Center.
b. The NASA OHP provides support and guidance to the Centers on health and wellness initiatives.
2.2.2.3 Process Description
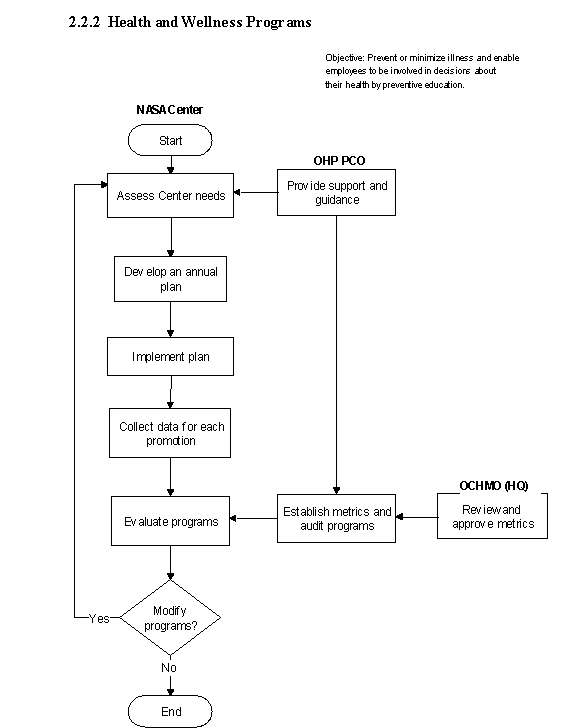
a. An assessment of the Centers' health promotion needs, based on demographics and risk factors, is completed.
b. An annual health promotion plan is developed based on the needs assessment and the available resources. When possible, health promotion initiatives are coordinated with national holiday observances.
c. Plans are implemented and data collected for each event.
d. The health promotion plan and data are evaluated. Evaluations include participation rates, abnormal findings and referrals, customer satisfaction, and changes in health status and health behaviors.
2.2.2.4 Flow Diagram
The flow diagram of this process is shown in Figure 2.2.2 at the end of this section.
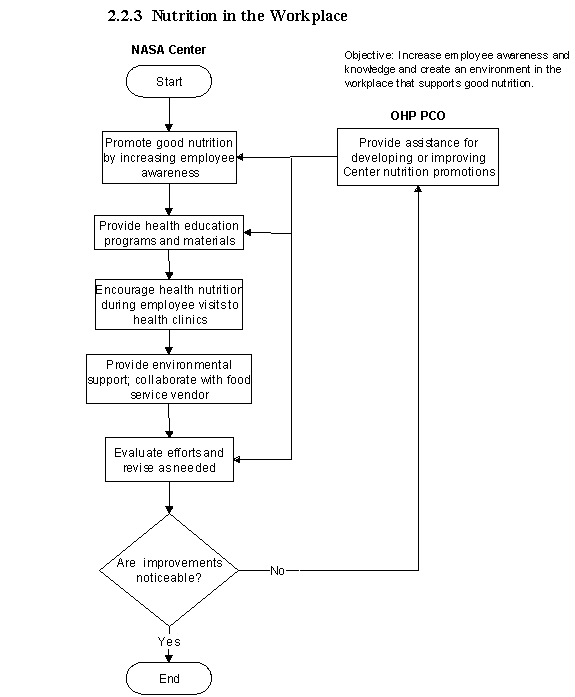
2.2.3 Nutrition in the Workplace
2.2.3.1 Introduction
a. Nutrition plays a major role in employee health and well-being. The goal is to increase awareness, knowledge, and create an environment in the workplace for NASA employees that support good health practices.
b. Creating an environment within the Centers that supports good health practices and good nutrition is important in improving employee health and well-being.
2.2.3.2 Responsibilities.
a. NASA Center Medical Directors are responsible for ensuring the implementation of nutritional awareness and education programs as well as advocating for environmental support in the workplace with the food service vendor.
b. The NASA OHP provides guidance and technical support for promoting nutrition at the NASA Centers. Technical support includes assistance to NASA Centers in providing employees with proper diet information, in the dietary control of diabetes, hypertension, and lipid disorders, and in the planning of cafeteria menu choices.
2.2.3.3 Process Description
a. Program Implementation
(1) Three levels of health promotion activities are recommended for implementing programs at NASA Centers. The first level is to increase awareness within the workforce of the impact nutrition has in maintaining good health and preventing disease. Secondly, provide Center employees with health education opportunities related to good nutrition that may result in behavioral changes. The final level is to encourage an environment that emphasizes good health practices by providing healthy food selections in cafeterias and vending machines.
(2) Formation of a Center employee committee to support health promotion activities should include representatives from different employee groups, including management and union personnel and the vendor contractor manager. The committee is responsible for tailoring health promotion activities to Center needs. It should develop a plan that includes program goals and objectives, planned activities, promotion strategies, an implementation schedule and metrics to be collected for program evaluation.
b. Environmental Support.
(1) Environmental support creates the structure needed for maintaining behavioral changes by ensuring the availability of healthy choices in the cafeteria and vending machines.
(2) Occupational Health Program staff can facilitate potential environmental changes with the contractor by working with the Food Service Vendor Contracting Officer.
(3) Food Service Contract Requirements
(a) Identify and provide a variety of low-fat, low-sodium, and high-fiber healthy choices in the cafeteria.
(b) Offer healthy choices for catered meetings or Agency sponsored events.
(c) Offer one healthy hot entre with a total fat content of less than 30 percent of total calories, saturated fat of less than 8-10 percent of calories, cholesterol of le , ss tha n , 150 milligrams, and sodium of less than 1000 milligrams.
(d) Provide nutritional labeling at the point of service for all hot entr?e selections including the total calories, percent of calories from fat, total fat, total saturated fat, and cholesterol.
(e) Label low-fat and high-fiber selections offered at salad, soup, and sandwich bars at the point of choice.
(f) Utilize products certified as Heart Healthy by the AHA.
(g) Modify recipes to lower content of total overall and saturated fat, cholesterol, and sodium.
(h) Modify food preparation by using cooking methods that do not add fat, e.g., baking, broiling, and boiling, trimming fat from meat, removing skin from poultry, and minimizing the use of gravy and sauces.
(i) Promote selection of healthy food choices by using a theme such as "Healthy Heart," "Just For You" or other nutritional campaigns.
(j) Post a permanent display of nutrition information including the Food Pyramid and Dietary Guidelines for Americans.
(k) Offer low-calorie, low-fat, and high-fiber snacks in the Centers' vending machines.
(4) Program Evaluation
Evaluating the impact of health promotion programs within the Agency is an essential component of a program. The short- and long-term program goals should be established in the planning stage.
2.2.3.4 References
a. NASA OHP Web site at http://ohp.nasa.gov/topics/nutrition
b. NASA Technical Bulletin "Promoting Nutrition in NASA Centers."
2.2.3.5Flow Diagram
The flow diagram for this process is shown in Figure 2.2.3 at the end of this section.
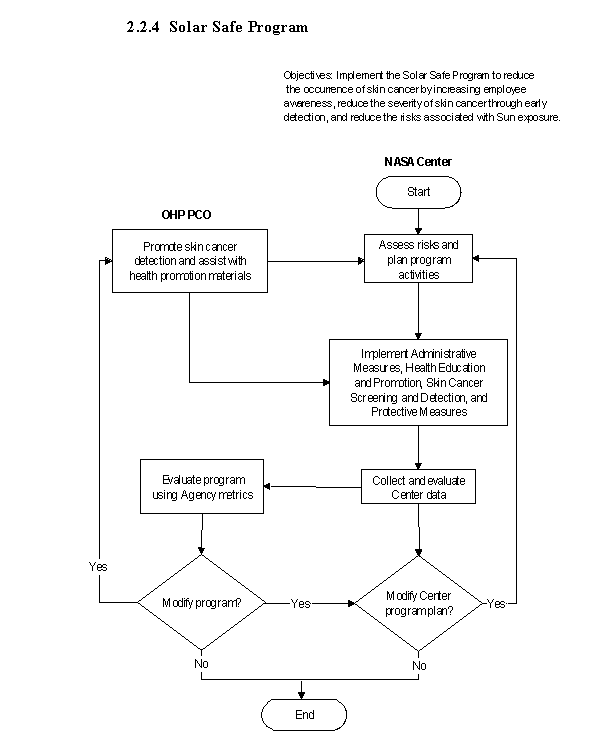
2.2.4 Solar Safe Program
2.2.4.1 Introduction
The Solar Safe Program is a preventive health initiative developed to address the risks associated with the development of skin cancer.
2.2.4.2 Responsibilities
a. The NASA OHP is responsible for providing health promotion materials, collecting Agency metrics and offering technical support. The OHP provides ongoing support with dermatology education programs, and screening and referral criteria.
b. The NASA Center Medical Directors are responsible for the implementation of the program. All NASA Centers must provide skin cancer screening as part of their medical services and cancer screening efforts.
2.2.4.3 Process Description
a. Reserved.
b. Reserved.
c. The program components implemented at the Centers are as follows:
(1) Administrative measures such as work schedule changes to reduce the amount and duration of exposure by limiting peak exposure between 10 a.m. and 2 p.m., thereby decreasing the potential risk of developing skin cancer.
(2) Health education and promotion activities to increase the awareness of potential risks associated with exposure to the Sun, employee education and prevention.
(3) Skin cancer screening and detection is offered to the NASA workforce in the form of general population screenings as a component of periodic physicals and medical surveillance exams.
(4) Protective measures include the use of personal protective gear such as hats and making sunscreen products available to reduce Sun exposure, ultraviolet sensor cards to increase employee awareness of harmful radiation and quantify exposure level for skin block protection.
d. Reserved.
2.2.4.4 References
a. NASA Technical Bulletin "Promoting NASA's Skin Cancer Prevention Program 'Solar Safe."
b. NASA OHP Web site at http://ohp.nasa.gov/topics/skin cancer
2.2.4.5 Flow Diagram
The flow diagram for this process is shown in Figure 2.2.4 at the end of this section.
2.2.5 Smoking Cessation
2.2.5.1 Introduction
a. Reserved.
b. The NASA OHP promotes smoking cessation using standard procedures across the NASA Centers to identify smokers, to assess smokers' interest in quitting smoking, and to provide useful smoking cessation information regarding available programs.
2.2.5.2 Responsibilities
a. NASA Center OHP health care professionals are responsible for establishing a smoking cessation program utilizing personnel from various occupational health disciplines and notifying the OHP that Center needs are met by locally available programs or that assistance is required.
b. The OHP supports ongoing efforts at NASA Centers to reduce the number of employees who smoke. It is responsible for providing information on how successful smoking cessation programs are established and maintained to NASA Center OHP health care professionals.
2.2.5.3 Process Description
a. Center health care professionals provide periodic assessments to the OHP on the progress and outcomes of the program, which are reflected in Agencywide assessments.
b. The OHP provides information on smoking cessation programs to health care professionals at NASA Centers to encourage them to establish or improve smoking cessation programs.
2.2.5.4 References
a. Executive Order 13058, Protecting Federal Employees and the Public From Exposure to Tobacco Smoke in the Federal Workplace, August 9, 1997.
b. Agency for Health Care Policy and Research, Clinical Practice Guideline No. 18, b.Smoking Cessation, 1996.
c. Public Health Service, Healthy People 2000: Mid
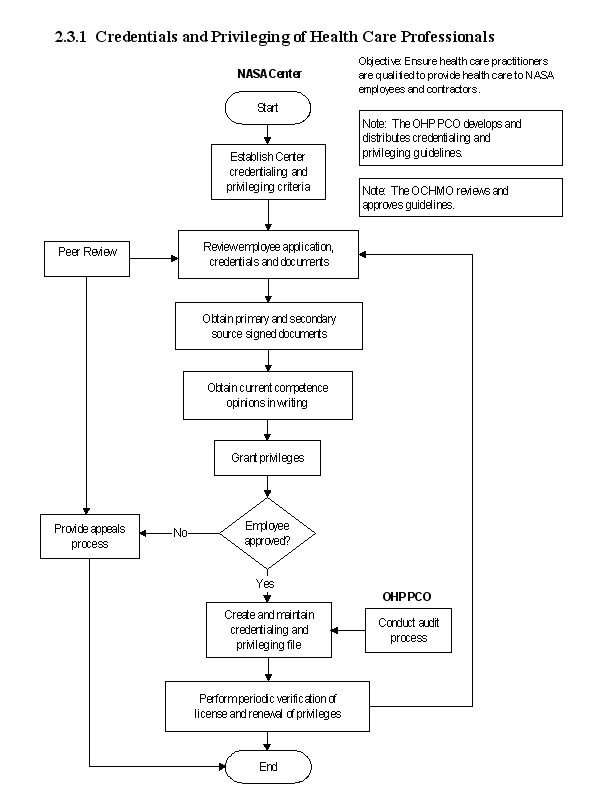
2.3.1 Credentialing and Privileging of Health Care Professionals
2.3.1.1 Introduction
a. The Credentials verification and privileging of health care staff is necessary both for ensuring the safety of employees and study volunteers and for delivery of quality health care. To facilitate NASA Center Occupational Health Program managers in performing these functions, the has developed a checklist that Occupational Health personnel may use to accomplish the task. The checklist is shown in Appendix B.
b. Several factors converge to direct that health care systems assure the existence of authentic practice credentials and proper privileging of health care professionals. The credentials verification process helps assure that the provider, in whose hands patients entrust their health and wellness, possesses current knowledge and appropriate skills. Privileging identifies and authorizes conduct of those procedural tasks for which providers must possess adequate skills in order to perform their responsibilities. Continuing education, relevant training, and skills utilization help to maintain competence, continuous improvement in efficiency, and effectiveness of health care. The use of credentialing and privileging augments the caliber of health care while minimizing the risk of untoward events. With these measures operative, NASA demonstrates its commitment to excellence, to employees, and to sustained high quality of health care.
c. Paramount in the processes of credentials and privileging are uniformly applied criteria for appointment, maintenance, and retention of currently knowledgeable, experienced, and competent health care professionals. Besides verifying essential information from authenticated primary sources, this will also require some degree of adaptation and customization to the many health care activities implemented at each NASA Center .
d. NASA credentialing and/or privileging criteria will be required of all licensed professionals involved in health care or human research by NASA or under contract to NASA, whether located physically on site at a Center or externally. Health care delivery will be interpreted as the supervision of, monitoring of, or direct delivery of health care to NASA astronauts, the employees, employee family members, visitors (in an emergency), or test/research volunteers. Such personnel include licensed independent practitioners (allopathic and osteopathic physicians, licensed psychologists who provide services without direction or supervision) and supervised health care providers (physician assistants, nurse practitioners, nurses). This system and these criteria have clear policies and procedures and well-defined roles and responsibilities for all participants . For the purpose of credentials verification, three levels of Health Care will be recognized are as follows:
(1) Supervision or research monitoring in a nondirect patient care role will require credentials verification and in some cases privileging,
(2) Direct patient/client care, counseling, or direct research monitoring will require both credentials verification and privileging, and
(3) Nonlicensed, supervised health care providers will require privileging for specific functions.
e. These stipulations do not apply to personnel at noncontract off-site facilities to which employee patients may be referred.
2.3.1.2 Responsibilities
a. Credentialing is managed by the CHMO. Center Chief Medical Officers/Medical Directors will implement these criteria for all civil service health care professionals and, together with technical and contracting personnel, assure that the same criteria are stipulated and affected for health care providers in all contracts procuring health care or human research services. The above are also responsible to ensure availability of adequate resources of time and training to maintain required licensure, certification, and privileges.
< processes. privileging and credentials Agency??s the with compliance assure to assess periodically implementation, their oversee guidelines, Agency promulgate establish will OHP NASA??s>
2.3.1.3 Process Description
a. Basic standards for the process are derived from the standards applied to hospitals and ambulatory health care facilities. The following four core criteria are :
(1) Current licensure.
(2) Relevant education, training, and/or experience.
(3) Current competence.
(4) Evidence suggesting the ability to perform requested privileges.
b. Information necessary to support items (1) and (2) will be obtained from primary sources, in written, signed documents, and in advance of appointment. Where telephonic or electronic means are employed to acquire information, explicit written verification of the source will be provided.
c. Upon request, NASA may privilege incumbent practitioners, who do not meet explicit credentials criteria, on the basis of their record of experience in a particular setting and of demonstrated proficiency in the procedure for which privilege is requested (grand-fathering). Each case will be considered individually and decided by peer representatives and if it involves licensed independent practitioners, the decision will be made through the Medical Policy Board.
d. Providers in advisory, supervisory, or research monitoring functions - in a nondirect care role - requiring a licensed health care professional (e.g., physician, nurse, dietitian) who may also be called upon to assist in an emergency.
(1) Shall provide evidence of the following credentials: Proof of professional education,evidence of specialty training, board certification if applicable and licensure to practice in a U.S. State or Territory.
(2) Shall maintain training in Basic Life Support (BLS) and the use of Automated External Defibrillator (AED).
(3) Shall meet other specific privileging or certification required by the assigned function.
(4) If functioning as a Medical Review Officer for NASA, shall be certified by a national certifying agency - American Association of Medical Review Officers (AAMRO) or American College of Occupational and Environmental Medicine (ACOEM).
e. Providers - in a direct care role - in health care delivery, counseling, or direct human research monitoring functions requiring a licensed health care professional (e.g., physician, psychologist, nurse) who are likely to be called upon to assist in an emergency are as follows:
(1) Shall provide evidence of the following credentials. Proof of professional education, evidence of specialty training, board certification if applicable, and licensure to practice in a U.S. State or Territory.
(2) Shall maintain certification in Advanced Cardiac Life Support (ACLS) and defibrillator use (AED and the specific defibrillator(s) in their care location).
(3) Shall meet other specific privileging or certification required by the assigned function (e.g., Medical Review Officer, sigmoidoscopy, conduct/monitoring of stress tests).
f. All supervised and non-licensed health care staff, such as clinic, emergency, or laboratory support personnel, on Earth or in space, performing or supporting procedures (e.g., electrocardiogram interpretation, audiological interpretation, pulmonary function testing) for which privileging must be granted in order to function in a direct-patient care setting are as follows:
(1) Shall be trained in BLS.
(2) May be required to obtain organizational "Certification" or "Licensing" after training in assigned procedures or functions for certain privileging (e.g., audiometric testing, X-ray technician functions, Breath Alcohol Testing (BAT)).
g. The complexities involved in Workers' Compensation Program cases may mandate specific training in this discipline; some States require certification (e.g., Florida ) to work in this arena. To assure competence and uniformity in managing these cases, credentials and privileging must be instituted.
h. Current competence will be attested by signed letters of informed opinion from specialty certification boards, peers of personal acquaintance, medical supervisors, academic program directors, or by demonstrated competency. These attestations will describe the applicant's clinical performance, clinical judgment, and technical skills (including operative procedures), discharge of professional obligations, and ethical demeanor. A search for any history of medical malpractice and of criminal record will be included, including a query of the National Practitioner's Data Bank.
i. Privileges will be delineated and granted in accordance with documented professional competence and facility support capability provided. The process will emphasize scrutiny and thoroughness prior to appointment. Continuous improvement of existing professional competence and of services offered will be sought. Where unacceptable performance must be confronted, the process will be fully documented and a fair hearing and redress assured with adverse decisions.
j. All credentials and credentialing information are subject to strict individual confidentiality. In the case of contractors, records of individual credentials verification and list of privileges shall be maintained by the hiring organization. Additionally a similar record shall be kept at the practicing organization with postings of relevant licensures at the practicing facility. All records containing credentialing information will be maintained in accordance with 5 U.S.C. 552a, the Privacy Act of 1974, as amended.
k. In the case of civil service personnel, records of individual credentials verification and list of privileges shall be maintained by the Office of the Chief Health and Medical Officer.
- References
a. The Credentialing Resource Center, Division of HCPro, Marblehead, MA.
b. 2000-2001 Comprehensive Accreditation Manual for Ambulatory Care, HR 7, JCAHO, Oakbrook Terrace, IL.
c. The National Committee for Quality Assurance (NCQA), Washington, DC.
d. The Credentials and Privileges Manual, The US Air Force, DoD, Washington, DC.
e. Procurement Notice, PN-97-58, NASA, Washington, DC.
f. NPD 8900.1E, Medical Policy Board.
2.3.1.5 Flow Diagram
The flow diagram for this process is shown in Figure 2.3.1 at the end of this section.
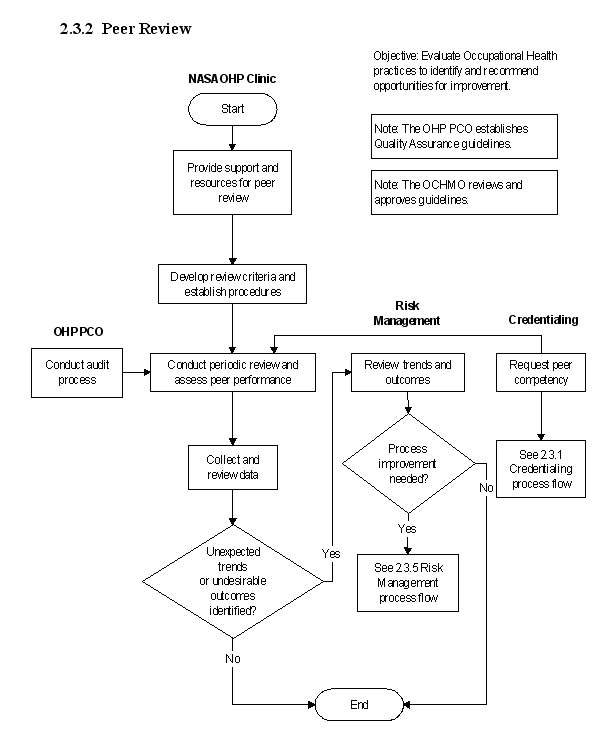
2.3.2 Peer Review
2.3.2.1 Introduction
a. Peer review is an important component of the quality process. The peer-review process evaluates the quality of care provided against established criteria based on standards and regulatory requirements. This process assists in the assessment of the competency of the healthcare professional and monitors treatment trends and outcomes. Results of the peer-review process provide valuable information and a critical link to the Quality Improvement and Risk- Management programs.
b. Standards of Care describe specific diagnostic or therapeutic maneuvers that should or should not be performed in certain clinical circumstances.
2.3.2.2 Responsibilities
a. The Center Medical Director is responsible for providing the resources and establishing the process for peer review at the NASA Centers.
b. The NASA OHP is responsible for establishing and maintaining the Agency guidelines on peer review. The NASA OHP conducts periodic audits of the peer- review process at the Center level.
2.3.2.3 Process
a. Elements of the process are outlined below.
b. Develop and implement an ongoing peer review process at each Center. The process ensures that the following recordkeeping requirements and established standards of care are met:
(1) Assigning and giving authority to personnel for the review process.
(2) Developing procedures for the peer review process.
(3) Conducting a review of medical records and assessing each one for completeness, timeliness, appropriateness of medical treatment and continuity of care.
(4) Collecting and evaluating data for trends and outcomes.
(5) Providing input to Quality Improvement and or Risk-Management personnel.
c . Require peer competency for the credentialing process that is based on information regarding the healthcare professionals clinical performance and skills and interpersonal relationships with peers and patients.
2.3.2.4 Flow Diagram
The flow diagram for this process is shown in Figure 2.3.2 at the end of this section.
2.3.3 Quality Improvement
2.3.3.1 Introduction
a. The NASA OHP is committed to continuous process improvements in the delivery of health services to civil service personnel, contractors, and visitors. The quality improvement process provides ongoing opportunities to improve processes, the quality of health care provided, and resolve problems. The evaluation process considers the administrative, clinical cost and patient outcomes with a focus on efficacy, effectiveness, and appropriateness of care and customer satisfaction. As a component of the Quality Assurance Program the quality improvement process is linked with the risk management and peer-review process.
b. Several terms used in describing quality improvement need to be defined. These include the following:
(1) Quality Improvement - An approach to the continuous study and improvement of the processes of providing health care services to meet the needs of individuals and others.
(2) Quality Assurance - The formal and systematic exercise of identifying problems in medical care delivery, designing activities to overcome these problems, and carrying out followup steps to ensure that no new problems have been introduced and that corrective actions have been effective.
(3) Standards of Care - Specific diagnostic or therapeutic maneuvers that either should or should not be performed in certain clinical circumstances.
2.3.3.2 Responsibilities
a. The Medical Director at each NASA Center is responsible for implementation and management of a quality improvement program.
b. The NASA OHP is responsible for establishing policy and guidelines for quality improvement.
2.3.3.3 Process Description:
Elements of the process include the following:
a. Developing a quality improvement plan as part of the overall Quality Assurance Program at each Center.
b. Assigning responsibility for the quality improvement process.
c. Reviewing recommendations for quality improvements received from risk management and peer review.
d. Defining the scope of care for the occupational health program.
e. Identifying quality care indicators such as patient satisfaction and outcomes of care.
f. Collecting, organizing and analyzing data.
g. Recommending and implementing actions to improve care.
h. Assessing the effectiveness of the actions and documenting improvement.
i. Communicating results to the staff.
2.3.3.4 Flow Diagram
The flow diagram for this process is shown in Figure 2.3.3 at the end of this section.

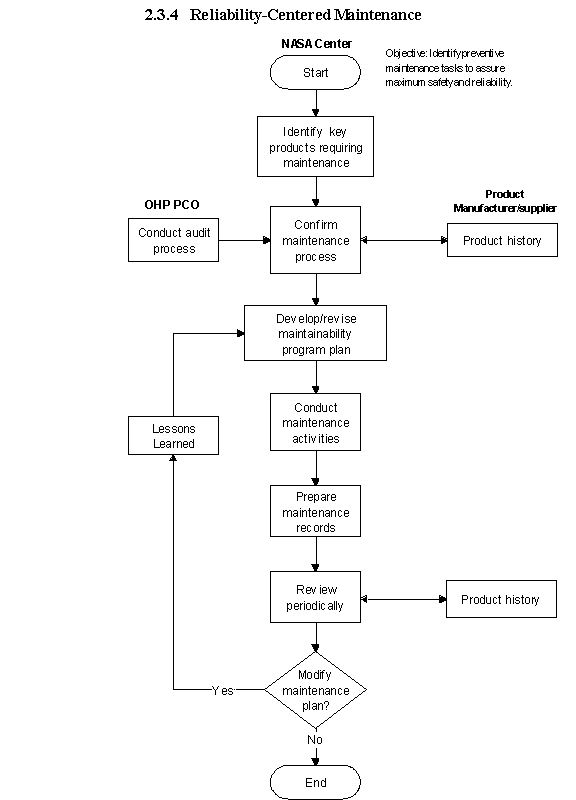
2.3.4 Reliability-Centered Maintenance
2.3.4.1 Introduction
a. The purpose of reliability-centered maintenance is to identify maintenance tasks to be performed periodically to sustain the maximum level of reliability and safety of the product and prevent downtime caused by unscheduled maintenance. The goal is to maintain a high state of readiness, and it is initiated as part of product design and development. The manufacturer normally provides a predetermined maintenance schedule. Most of the improvements for operational Occupational Health products occur in safety and operational reliability.
b. Reliability is the likelihood that a piece of equipment is in adequate condition to carry out its intended functions and that it will not experience failure during a given period.
c. Key equipment includes emergency equipment (e.g., AED, other defribrillators, respirators), all vital equipment for OSHA compliance (e.g., samplers, pulmonary function testers), high-value equipment (e.g., x-ray units, electrocardiographic equipment), and equipment necessary to maintain normal operations in accordance with the Safe Medical Device Act.
2.3.4.2 Responsibilities
Center Medical Directors are responsible for the development of Reliability-Centered Maintenance programs specifically for medical equipment (e.g., crash carts and defibrillators).
2.3.4.3 Process Description
a. Elements of the process include the following:
(1) Develop a maintainability program plan that is used to collect maintenance information on all key equipment in one location. The information can be in a tabular or outline format if applicable.
(2) Equipment section list s key equipment to be covered and identifiers such as the date of manufacture (if available from the equipment) and the model number.
(3) Reference section includes new information or alerts from the manufacturer or regulatory agency and includes location of any other equipment information such as manuals, circuit diagrams, specifications.
(4) Technical communication section includes the manufacturer and manufacturer contact number and Center equipment maintenance contact number.
(5) The maintenance concept section includes the basic maintenance requirements and organizational responsibility . This area should include period of equipment test (daily, weekly, yearly), who should perform the test or maintenance, the criteria for a successful test, and who should review any deviation from a normal result.
(6) Policy and procedure section outlines the actions to be taken if a deviation from normal occurs but does not duplicate test/maintenance procedures available in the equipment manual.
(7) The organizational interface section addresses the lines of communication among the operational organization, the maintainability organization, and any other organization involved.
(8) Update and resolve equipment problems and keep maintenance history for inclusion in the Reference section . Maintenance personnel contact with the manufacturer can obtain information on updated maintenance and test procedures, history of any product or component recalls, and perhaps reliability information and from periodic company maintenance, such as the top three components most frequently replaced and average time of replacement.
b. Information in history should include event, date of discovery, safety risk (none, low, medium, or high), downtime and action required.
c. Pro vide lessons learned concerning any new safety or reliability information to NASA and to the office responsible for maintaining Center Lessons.
2.3.4.4 Flow Diagram
The flow diagram for this process is shown in Figure 2.3.4 at the end of this section.
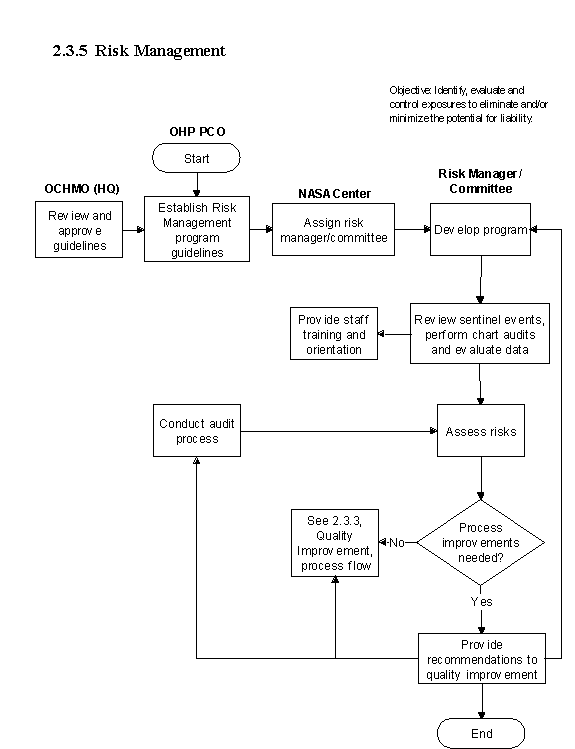
2.3.5 Risk Management
2.3.5.1 Introduction
a. The Occupational Health Risk Management Program seeks to identify, evaluate and control exposures to eliminate and/or minimize the potential for liability. Effective risk-management programs at each NASA Center help to reduce the potential risk of injury to civil service personnel, contractors and visitors as well as maintain public confidence for the Agency. Risk management activities include administrative and clinical aspects of the NASA OHP. The risk-management activities and feedback are linked in the con , tinuous Qualit y Improvement , p rocess.
b. Several terms used in risk management need to be defined. They include the following:
(1) Risk Management - The identification, evaluation and reduction of risk of injury follows the ASI Safety and Health Hierarchy which prioritizes safety efforts for the public, astronauts, pilots, NASA workforce, and high-value equipment and property.
(2) Sentinel Event - An unexpected occurrence or variation involving death or serious physical or psychological injury, or the risk thereof.
(3) Root Cause Analysis - A process for identifying the basic or causal factor(s) that underlie variation in performance, including the occurrence or possible occurrence of a Sentinel Event.
2.3.5.2 Responsibilities
a. Center Directors are responsible for ensuring the development and management of a Centerwide Risk-Management Program. The Center's risk based acquisition management program may require technical consultation from medical, radiological health, and environmental/industrial health in addition to Safety, security, and export control input.
b. Center Medical Directors are responsible for the development and management of the medical portion of the center's Risk-Management Program.
c. Center Environmental Health Managers are responsible for the development and management of the environmental health portion of the center's Risk Management Program.
d. The NASA OHP is responsible for establishing guidelines for OHP risk management and periodically auditing the program in place at each Center.
2.3.5.3 Process Description
- Elements of the process include the following:
(1) Assigning an individual or a committee to be responsible for the program.
(2) Developing a risk-management program at each NASA Center .
(3) Ensuring adequate coverage during and after normal clinic hours.
(4) Conducting regular audits.
- Ensuring effective risk management by reviewing program components such as the following:
(1) Data trends.
(2) Complaints.
(3) Incidents.
(4) Deaths, trauma, or adverse events.
(5) Staffing levels, skill mix, and education.
c. Ensuring orientation for new staff members that includes reviewing current trends, means of minimizing risk and quality improvement initiatives.
d. Providing education on risk-management activities to all staff.
e. Reviewing any Sentinel Event, conducting analysis of the root cause, and recommending corrective action.
f. Managing incident reporting.
g. Providing recommendations to Quality Improvement.
h. Ensuring that both health a nd safety issues are addressed and coordinated.
- References
a. NPR 7120.5A, NASA Program and Project Management P roce sses and Requirements, April 3, 1998 .
b. NPR 8715.3, NASA Safety Manual, January 24, 2000.
c. Procurement Notice 97-58, Risk Management, November 22, 2000.
2.3.5.5 Flow Diagram:
The flow diagram for this process is shown in Figure 2.3.5 at the end of this section.
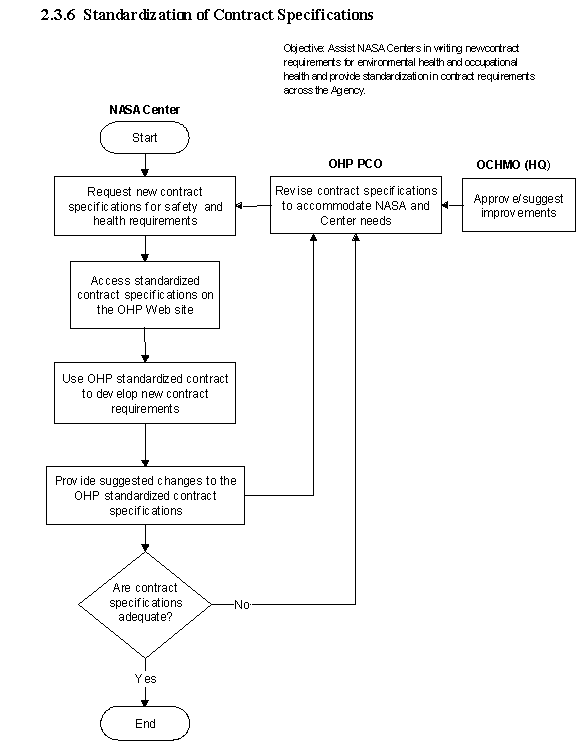
2.3.6 Standardization of Contract Specifications
2.3.6.1 Introduction
a. The NASA OHP recognizes that each NA SA Center manages its occupational health programs differently. At some Centers, the environmental health function may be completely performed by NASA personnel while other Centers may have this function outsourced to a contractor. The occupa tional medicine function at most NASA Centers is provided by contractor and managed by NASA personnel. Outsourcing of this function has resulted in the establishment of stand-alone contracts at the individual Centers that do not provide continuity or equality of services across the Agency. The NASA OHP examined support contracts from several Centers and adopted the best elements of each contract for inclusion in one document.
b. This document is available as a reference guide to Center personnel who are preparing the requirements for environmental health and occupational medicine support contracts at the Centers. This document does not supersede or impose new requirements for contracts but only serves as a guideline for developing and documenting requirements. It may initiate the development of contract equality and services across the Agency.
2.3.6.2 Responsibilities
a. The NASA Centers are responsible for ensuring that their environmental health and occupational medicine personnel are equipped with resources necessary to perform their jobs in an effective manner.
b. The NASA OHP is responsible for revising this document and continually updating the Web-based desktop guide to ensure that it is current with new regulations or significant issues and incorporates suggested improvements from Agency or Center management.
2.3.6.3 Process Description
a. The specific areas covered for environmental health are as follows:
(1) Establishing industrial hygiene program services.
(2) Establishing health physics program services.
(3) Establishing environmental sanitation program services.
(4) Maintaining a material safety data sheet program.
(5) Supporting emergency response teams.
(6) Acquiring laboratory accreditation.
(7) Managing an asbestos abatement program.
(8) Qualifying for or acquiring personnel licensure, certification, training, and experience.
b. The specific areas covered for occupational medicine are as follows:
(1) Providing emergency medical services.
(2) Defining staff positions, duties, and required qualifications.
(3) Establishing hours of operations.
(4) Establishing the scope of services required.
(5) Defining health maintenance examination requirements.
(6) Maintaining medical records.
(7) Maintaining an EAP.
(8) Defining report requirements.
(9) Managing health promotion activities.
(10) Providing medical consultation.
(11) Maintaining a health fitness center.
(12) Providing medical advice to committees and programs.
(13) Maintaining medical equipment and supplies.
- References
a. NASA OHP Web site - http://ohp.nasa.gov/, for standardized contract specifications.
b. Department of Defense (DOD) Standardization Program - http://www.dsp.dla.mil/, for standardization of contracts.
c. Procurement Notice 97-58, Risk Management, November 22, 2000.
d. Ames Research Center Statement of Work for Emergency Medical Services and Occupational Health (No. 2-35338, January 26, 1993).
e. Dryden Flight Research Center Statement of Work for Occupational Health Services (NAS2-14002, April 4, 1994).
f. Glenn Research Center Statement of Work for Environmental Health Services.
g. Glenn Research Center , Lewis Field, Performance Work Statement for Health Screening Program, Physical Fitness Program, and Occupational Medicine Program (May 2, 2000).
h. NASA Headquarters Statement of Work for Comprehensive Occupational Health Program.
i. Jet Propulsion Laboratory Charter for Occupational Health Services - Section 1980 and Statement of Work for Occupational Health Services (NAS&-1260, February 1997).
j. Johnson Space Center Statement of Work for Environmental Health & Occupational Medicine Services.
k. Kennedy Space Center Statement of Work for Environmental Health & Occupational Medicine Services.
l. Kennedy Space Center Statement of Work for Medical and Environmental Services (NAS10-99001).
m. Langley Research Center Statements of Work for Environmental Health Services and for Industrial Hygiene Services.
n. Marshall Space Flight Center Performance Work Statement for Environmental Health and Occupational Medicine Services (NAS8-97321).
2.3.6.5 Flow Diagram
The flow diagram for this process is shown in Figure 2.3.6 at the end of this section.
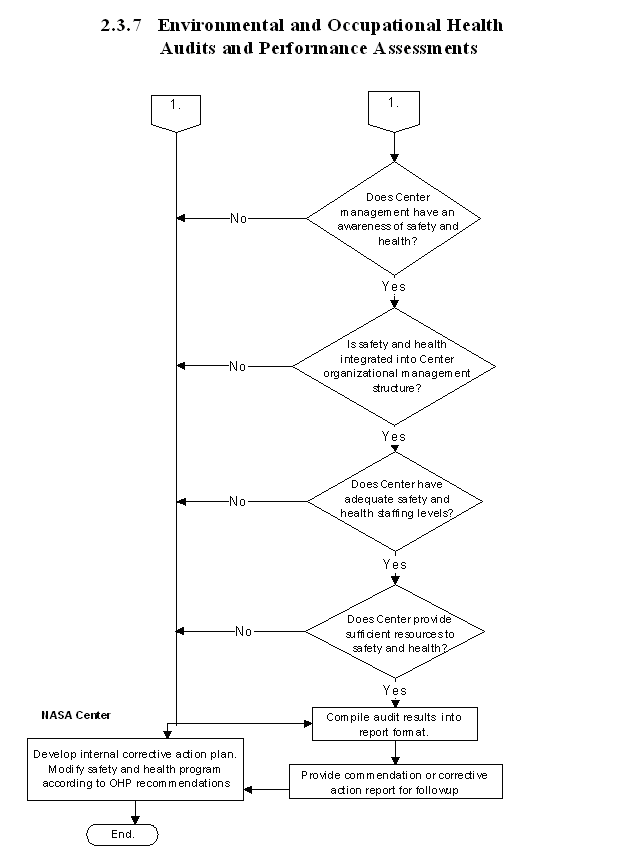
2.3.7 Occupational and Environmental Health Programs Performance Assessment
2.3.7.1 Introduction
The NASA OHP is committed to providing its employees with a safe and healthy workplace. To ensure that this commitment is implemented, the OHP has developed an audit process for the environmental health and occupational medicine program to ensure a successful effort.
2.3.7.2 Responsibilities
a. NASA Center personnel are responsible for implementing both environmental health and occupational medicine programs at their Centers.
b. The NASA OHP is responsible for assessing the effectiveness of the Agency's environmental health and occupational medicine programs at NASA Centers. To carry out this responsibility, it conducts continuous improvement audits of NASA Centers' environmental health and occupational medicine programs on a rotating basis or when specifically requested by an individual Center.
2.3.7.3 Process Description
a. The continuous improvement audit process involves examining a variety of environmental health and occupational medicine processes at NASA Centers.
b. Examples of the environmental health elements of this process are as follows:
(1) Management Leadership and Employee Involvement
(2) Management Leadership
(3) Employee Participation
(4) Implementation
(6) Contractor Safety and Health
c. Workplace Analysis
d. Health Hazard Prevention and Control
(1) Health Hazard Prevention and Control
(2) Health Hazard Control
(3) Medical Program
- Emergency Environmental Health Response
- Environmental Health Training
g. Examples of the occupational medicine elements of this process are as follows:
(1) Medical
(2) Center Demographics
(3) Employee Rights and Organizational Ethics
(4) Quality of Care
(5) Center Health Promotion and Wellness
(6) Organizational Performance
(7) Leadership
(8) Management of Human Resources
(9) Management of Information
(10) Surveillance, Prevention, and Control of Infection
(11) Management of the Medical Care Environment
h. Workers' Compensation
- Fitness Program and Facility
- Employee Assistance Program
k. When the OHP conducts an assessment, the necessary personnel, documentation, and resources are made available to the team of visitors. The OHP provides an exit briefing for Center personnel before leaving, and a written report of its findings is submitted to Center personnel within a few weeks after the visit.
2.3.7.4 Flow Diagram
The flow diagram for this process is shown in Figure 2.3.7 at the end of this section.
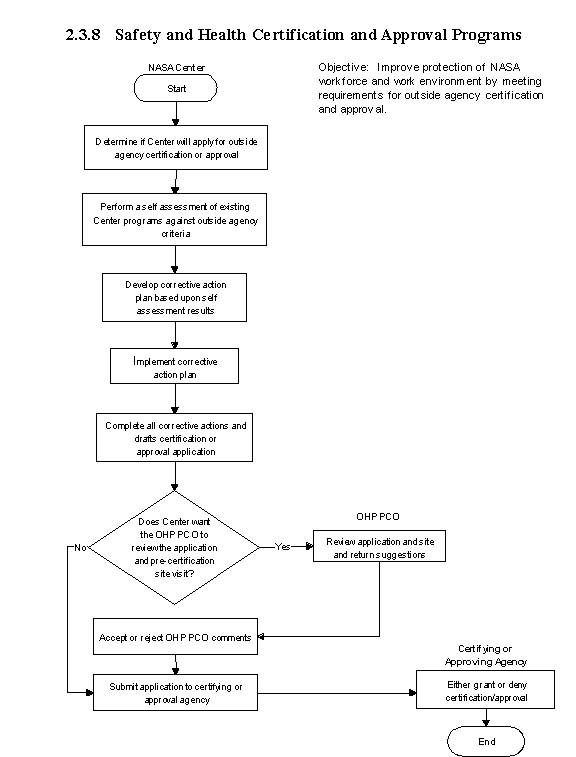
2.3.8 Safety and Health Certification and Approval Programs
2.3.8.1 Introduction
a. OSHA has developed voluntary guidelines on the management of safety and health programs. The International Organization for Standardization (ISO) has also developed a standard on environmental management (ISO 14001) and plans to publish a standard on occupational safety and health management (ISO 18000). Both systems are based on the concept of managing occupational safety and health protection of workers and the environment rather than just attempting to comply with Government standards.
b. Both of these programs are similar in approach. They examine the same program elements and also produce a similar goal of recognition of exemplary safety and health programs. However, there has been a delay with the finalized development and release of the ISO 18000 standard. It is, therefore, recommended that if a NASA Center is going to pursue the implementation of an occupational safety and health management system, the established OSHA guidelines should be utilized. If the ISO 18000 certification is required by the NASA Center when the standard is eventually published, the task will be made easier by already having an established occupational safety and health management system in place. Based upon these reasons, the rest of this section focuses on OSHA Voluntary Protection Program (VPP).
c. The VPP provides a venue for establishing a cooperative agreement among management, labor, and OSHA. From this agreement, a proactive approach to the management of safety and health issues is achieved, and benefits, such as reduced injury and illness rates, decreased insurance premiums, and increased quality, productivity and employee morale, can be realized. The Agency fully supports the adoption and implementation of these procedures at its Centers.
- Responsibilities
a. The NASA Center is responsible for implementing the requirements of the guidelines and for providing any additional funding required to support this program effort.
b. The OHP OCP is responsible for providing assistance to the NASA Centers with their approval efforts. Assistance may include application review, on-site readiness reviews, coordination with certifying agencies and lending/providing of subject reference material to the Centers' implementation team.
- Process Description
a. The VPP has three program levels of achievement which include the following:
(1) Star, the highest level of achievement which denotes a site that is not only successful in implementing a comprehensive safety and health program but one that also goes beyond compliance and strives for a leadership role.
(2) Merit, the recognition granted for sites that have demonstrated both the desire and the ability to meet the requirements of Star but have not quite achieved it.
(3) Demonstration the recognition given to sites that are unique situations not covered by Star requirements, such as nuclear power plants. OSHA will examine four areas of a site's safety and health program during a site visit. They are as follows:
(a) Management leadership and employee involvement.
(b) Worksite analysis.
(c) Hazard prevention and control.
(d) Safety and health training.
b. During a site visit, OSHA will not only be looking for evidence of documented programs, but also, and most importantly, subjective evidence that the programs are implemented. Employee involvement in the VPP effort is critical, and at most successful Star sites, the entire effort is run by employees with site safety and health personnel acting as consultants to the effort.
c. The time required for a facility or NASA Center to become certified varies from as little as 6 months to 2 years and is largely determined by the quality of existing programs and the commitment of both Senior Management and the employees.
2.3.8.4 References
OSHA website at http://www.osha.gov/oshprogs/vpp
2.3.8.5 Flow Diagram
The flow diagram for this process is shown in Figure 2.3.8 at the end of this section.
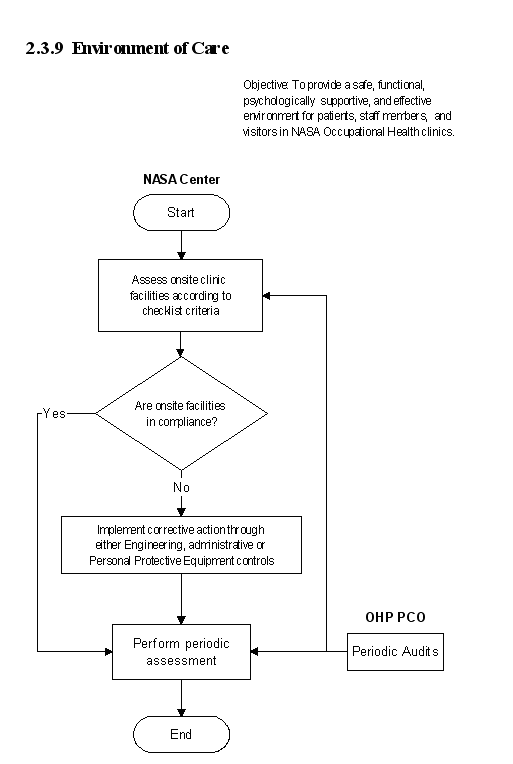
2.3.9 Environment of Care
2.3.9.1 Introduction
a. The NASA OHP is committed to ensuring the delivery of quality health services at NASA Center Occupational Health clinics. In order to facilitate this philosophy, the has developed a checklist that Occupational Health personnel may use to gauge the current status of their clinical facilities against Joint Commission on Accreditation of Health Care Organizations (JCAHO) Environment of Care (EOC) guidelines. The checklist is shown in Appendix C.
b. The goal of the Environment of Care process is to provide a safe, functional, supportive, and effective environment for patients, staff members, and other individuals in the clinic. This is crucial to providing quality patient care and achieving good outcomes. Achieving this goal depends on performing the following:
(1) Strategic and ongoing master planning by management for space, clear circulation of occupants, equipment, supportive environment, and resources needed to safely and effectively support the services provided.
(2) Educating staff about the role of the environment in safely, sensitively, and effectively supporting patient care.
(3) Developing standards to measure staff and clinic performance in managing and improving the environment of care.
(4) Implementing plans to create and manage the health unit's environment of care.
2.3.9.2 Responsibilities
Each NASA Center is responsible for the overall safety of its facilities including on-site occupational health clinics. In support of this task, the OHP offers this document to Center personnel who wish to assess their clinic's facilities against JCAHO criteria. The checklist is voluntary and not all inclusive of all JCAHO guidelines. If a Center wishes to pursue formal accreditation, it is advisable to consult the complete JACHO accreditation criteria.
2.3.9.3 Process Description
a. "Environment of Care" refers to a variety of "key elements and issues" that contribute in creating the way the space feels and works for people, patients, visitors, and staff experiencing the clinics healthcare delivery system. Certain key elements and issues that can be significant in their ability to positively influence patient outcomes and satisfaction are as follows:
(1) Light (both natural and artificial).
(2) Orientation and access to nature and the outside.
(3) Clarity of access (both exterior and interior circulation).
(4) Physical cues to navigating through the inside and around the outside of the clinic.
(5) Control.
(6) Privacy (visual, auditory, and document).
(7) Order.
(8) Space size and configuration appropriate and consistent with the clinical philosophy.
(9) Color compatability.
(10) Security assurance.
(11) Convenient patient flow and efficient layouts that support staffing and overall functional operation.
b. These elements when appropriately managed, create welcoming, comfortable environments that support and maintain patient dignity and allow ease of interaction, reduce stressors, and encourage patient/employee participation in the delivery of care. Additionally, by incorporating the management of these elements in standardized processes and procedures, the clinic's will realize the following:
(1) Reduced environmental hazards and risks.
(2) Reduced accidents and injuries.
(3) Safe conditions for patients, visitors and staff.
c. The checklist will examine the five functional areas that make up JCAHO's Environment of Care Guidelines.
d. Planning & Design
Compliance with the National Fire Protection Association (NFPA) Life-Safety Code is essential when constructing new or modifying existing facilities. This section of the checklist seeks to determine if the clinic's structure is built to code.
e. Implementation
According to the Environment of Care requirements, the clinic should have documented programs that address the following:
(1) Safety Management.
(2) Security Management.
(3) Hazardous Materials.
(4) Hazardous Waste.
(5) Emergency Preparedness.
(6) Life Safety Management.
(7) Medical Equipment.
(8) Utility Systems.
f. The checklist can identify program areas where clinic programs may be lacking.
g. Evaluation
This section focuses on the calibration and maintenance records of medical equipment and critical utility systems.
h. Orientation and Training
Environment of Care requirements specify that the clinic staff shall be educated about the role of the environment in safely and effectively supporting patient care. The education and training should include information about the physical characteristics and the processes for monitoring and reporting on the health care environment. This section of the checklist seeks to determine if this information is provided.
i. Environment
This section's goal is to give an appraisal of the tangible assets of the facility . Headings under this section include the following:
(1) Building and Grounds.
(2) Exiting.
(3) Emergency Power.
(4) Medical Gases.
(5) Central Gas Supply Systems.
(6) Compressed Air Systems.
(7) Central Vacuum Systems.
(8) Construction/Detail.
(9) Fire Protection/Life Safety.
j. Although the tried to be make this section as comprehensive as possible, differences in facilities, operations, geographic location and municipal/Center requirements, deviations may be necessary to effectively utilize this section. Note: Determinations on deviations from this section must be made by qualified personnel.
2.3.9.4 References
a. NASA Standard 8719.11, Safety Standard for Fire Protection.
b. NPR 8820.2c, Facility Project Implementation Handbook for Technical Guidance.
2.3.9.5 Flow Diagram
The flow diagram for this process is shown in Figure 2.3.9 at the end of this section.
2.4.1 Employee Medical Records
2.4.1.1 Introduction
a. An individual medical (health) record shall be established and maintained on every employee at the first encounter with an Occupational Health clinic. Medical records must be safeguarded, maintained and dispositioned in accordance with NPR 1441.1, NASA Records Retention Schedules. Such a record shall include any or all of the following:
(1) Reference and interim medical history and progress notes.
(2) Results of examinations by employee health staff.
(3) Treatments, medications, and immunizations prescribed or given.
(4) Medical consultations.
(5) Reports to and from physicians and other health care providers.
(6) Findings of fitness-for-duty examinations according to job requirements.
(7) Results of ret , urn-to-work examinations as required from hospitalizations of extended medical leave.
(8) Reports of job-related injury and illness.
(9) Occupational or accidental exposures and incidents.
(10) Environmental hazards/conditions of the work place related to health.
(11) Related laboratory test data.
(12) Biologic monitoring data.
(13) Required signed informed consents authorizing diagnostic and treatment procedures.
(14) Certain health related information obtained by questionnaire.
(15) Certain health insurance records.
(16) Specific job training completed.
b. Medical records require extreme care for their security and are subject to strictest confidentiality against unauthorized access or disclosure. Some types of health information must be disclosed by law. For example, Occupational Safety and Health Administration (OSHA) and Workers' Compensation (WC) require reporting of occupational injuries and illnesses. Sources of all information must be clearly identified by name, initials, or organization. Health records are never altered; when necessary, corrections are properly annotated, dated, initialed, and explanation given.
c. On the other hand, patient/employee access to their own health record is nearly always assured (except in certain situations; for example, psychiatric conditions, where an attending physician thinks knowledge of such information would prove detrimental to the patient's/employee's h , ealth).
d. Certain types of health-related information are so potentially vulnerable to misuse against patients/employees, e.g ., Employee Assistance Program (EAP) records, Human Immunodeficiency Virus (HIV) tests, that they must be kept in wholly separate record systems or coded to preclude direct identification with the patient/employee.
e. All records will be maintained in accordance with 5 U.S.C. 552a, the Privacy Act of 1974, as amended, and in accordance with the restrictions in the Health Information Management System (HIMS) in NASA's "Privacy Act; Annual Notice and Amendment to Systems of Records," published in the Federal Register.
2.4.1.2 Responsibilities
a. Each NASA Center Occupational Health Facility generates, maintains, and manages the employee health record. The record is subject to all provisions described above and any other legally valid determinations. The Center may not arbitrarily manage, disclose contents, or dispose of this record.
b. The Center Medical Director is responsible to ensure that Health Care Professional opinions provided by the health facility and required by regulations do not violate the employee's confidentiality . This responsibility also extends to ensuring confidential performance of drug, HIV, and bloodborne pathogen tests and that records are confidentially maintained, and any fax or electronic receipts of test results do not have public access. There is also responsibility to make records available for Quality Control review by the OHP when requested during a site audit.
c. The NASA OHP at least annually requires certain statistical information which must be derived from employee health and ancillary records . The may also access health records for purposes of quality assessment and planning towards practical uniformities and future automations. Patient confidentiality will always be maintained .
2.4.1.3 Process Description
a. The health record is one of the most diverse documents in common use today, from its content, its derivation, its form, and its organization. It is an absolute essential in the delivery of health care. And though it has universally recognizable elements, it follows no particular consistency in format. The health record continues to grow with little opportunity for compression.
b. To the extent that much of its content is not in digital computer convention, retrieval of some information may be cumbersome and slow. Moreover, since much of a medical record continues to be generated in handwritten format, readability (legibility) frequently suffers. These records are subject to random audit and should have periodic analysis for improving functionality as well as selected case review for enhancing the quality of health care delivered. The health record is really the major documentation of health care service rendered.
c. Because of the above and other concerns, the health record has been a prime target for some wholesale improvement with new concepts, technologies, and a substantial augmentation by automation. Structuring the retrieval by a problem-oriented record or computerization is strongly encouraged.
2.4.1.4 Flow Diagram
The flow diagram for this process is shown in Figure 2.4.1 at the end of this section.

2.4.2 Workplace Assessment, Hazard Analysis and Personal Exposure Records Management
2.4.2.1 Introduction
NASA Center OHP personnel generate a multitude of written and electronic records in support of their assigned job functions. These records, for example, include Area and Facility Inspections, Process Assessments, Monitoring Data, Corrective Action and Survey Reports. It is required that NASA properly manage these recor ds across the Agency in a uniform manner to meet regulatory requirements. To support this intent, the Agency has developed NPR 1441.1C, Records Retention Schedules. T he OHP fully suppor ts the utilization of these schedules by a ll NASA Center OHP pe rsonnel.
2.4.2.2 Responsibilities
a. NASA Centers are responsible for generating and maintaining occupational and environmental health records according to the Agency's published guideline. They also make these records available for inspection by OHP or OSHA personnel when requested during a site visit or compliance inspection.
b. The OHP is responsible for assessing the quality and consistency of these records.
2.4.2.3 Process Description
The workplace assessment/hazard analysis/personal exposure records management process shall follow the schedules developed in NPR 14 , 41.1C. Examples of the records covered by these schedules are as follows:
(1) Injury and illness reporting.
(2) Air sampling data.
(3) Area inspection data.
(4) Physical examination and healthcare data.
(5) Industrial hygiene reports.
(6) Health physics surveys and reports.
(7) Special permits.
(8) Construction and design recommendations.
- References
NPR 1441.1C, Records Retention Schedules, March 1997.
2.4.2.5 Flow Diagram
The flow diagram for this process is shown in Figure 2.4.2 at the end of this section.
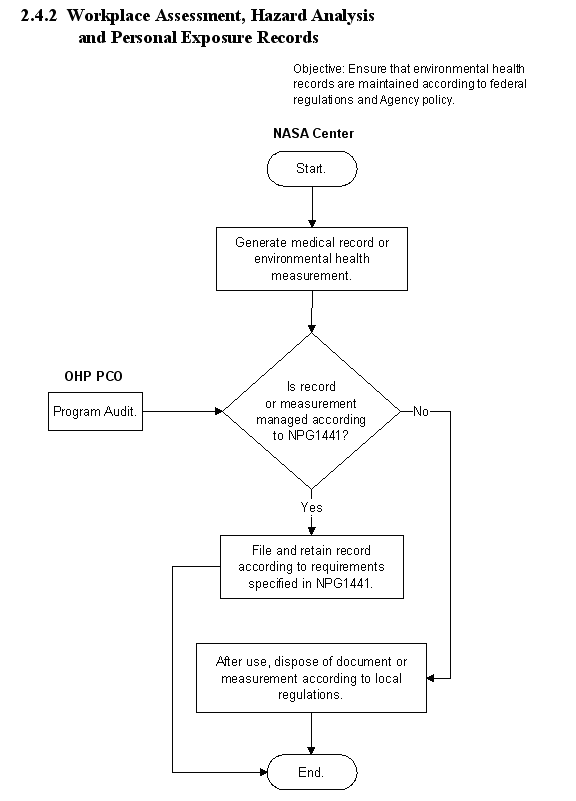
3.1.1 Disease/Injury: Prevention, Screening, and Early Detection, and Mitigation of Adverse Effects
3.1.1.1 Introduction
In the occupational setting, preservation of a healthy workforce is a priority equal to assuring a safe workplace, and in many ways the two are integrally related. NASA's workforce is it greatest strength. Efforts must be directed toward keeping members of the workforce healthy, safe and productive. From a management perspective, the operative concept is prevention. This not only is the least costly in terms of resources expended, but also minimizes the toll in lost work and human suffering.
3.1.1.2 Responsibilities
a. Real and enduring success in maintaining individual as well as corporate health depends upon a continuum of responsibility. Good health results when a culture recognizes the distinctive value of health and is dedicated to its achievement. Prevention begins with the individual.
b. The NASA Center OHP has overall responsibility for maintaining health and safety but the primary responsibility lies at each Center. Prevention really begins as a mind set. OHP personnel at all NASA Centers must think ahead to anticipate hazards, modify processes, and take actions to forestall harmful and injurious conditions and events. This applies to workplace and to worker, and it must be a continuous process.
c. Center OHP personnel must provide preventive services through medical surveillance, health and wellness promotions, immunizations, sanitization of food services, monitoring water supply and control of chemical and physical hazards.
d. The OHP assures implementation of relevant NPD's and regulatory requirements and provides functional management and guidance to health personnel at NASA Centers. It assists NASA Centers in selecting and acquiring resources, contributes to dissemination of information and training of personnel, and helps achieve Aagency-wide uniformity, consistency, and quality.
3.1.1.3 Process Description
a. Successful preventive occupational health services begin with a comprehensive assessment of workplace hazards and adverse environmental conditions. Coincident evaluations of current programs of prevention and of areas requiring preventive emphasis are integrally accomplished. Efficacy of preventive program implementations is determined by metrics appropriately selected to monitor relevant injuries and illnesses as well as achievement of goals and indicators of trends. Critical to such metrics are baseline determinations, and many employees must undergo limited or complete medical evaluations (often termed "fitness for duty") prior to and during their work in potentially hazardous conditions. Medical monitoring examinations are performed on employees in an established medical surveillance program. Timeliness of reporting and response are critical to eliminating/minimizing injurious effects of hazards and contraction of illnesses. The OHP encourages establishing and maintaining working relationships between OHP PCO personnel, occupational health management teams, and health professionals at each NASA Center. The work of occupational health preventive services is implemented and enhanced by onsite visits and audits, planning and training colloquia, management and professional interchange, and instant two-way communication by the latest technologies, including an active NASA OHP Web site.
b. Besides gathering information specific to a given NASA Center, overall Agency statistics are prepared and compared with those of similar industries and settings. Effective preventive health is allied to safety matters and management interests, such as productivity, lost time, workers' compensation, and program costs. The process of benchmarking, always seeking the best and improving status quo, is continuously applied where deemed beneficial. Emphasis on any preventive program effort must match priority and available resources.
3.1.1.4 Flow Diagram
The flow diagram for this process is shown in Figure 3.1.1 at the end of this section.
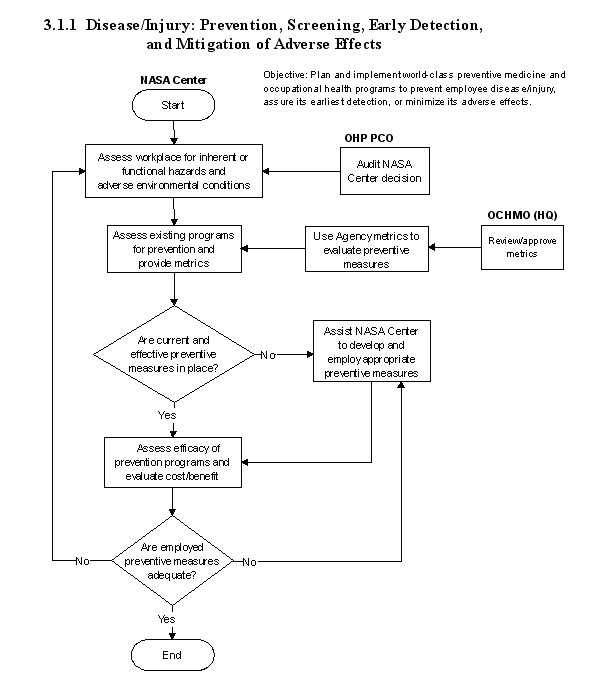
3.1.2 Medication Management
3.1.2.1 Introduction
a. In order to ensure the health and safety of its employees, the Occupational Health clinics must follow best practices and comply with regulations in managing onsite use of medication.
b. Policies and procedures for the control, accountability, and security of all drugs used in Center Occupational Health Clinics must be in place. The clinics must also ensure the competency of their professional staff responsible for administering medications. Additionally, emergency readiness standards and plans are a critical component to the medication management program and must be in place. To facilitate implementation and assessment of this function, the PCO has developed a checklist that Occupational Health personnel may use to assess their program. The checklist is shown in Appendix D.
3.1.2.2 Responsibilities
a. Center Chief Medical Officers/Medical Directors ensure that management of medications in the occupational health facility provides the resources necessary to meet the scope of services provided, and complies with federal, state and local regulations. They also ensure that the program and is administered by competent health care personnel.
b. The OHP is responsible for conducting periodic audits of the Center's medication management process.
3.1.2.3 Process Description
a. Administration - Regulations
Compliance with Federal and State regulations is essential when managing medications in the workplace. At NASA Occupational Health clinics, the Medical Director assumes responsibility for the management of the medication program. Guidelines for medication management must comply with the following:
(1) State Nurse Practice Act (defines the scope of nursing practice).
(2) State Medical Practice Act (addresses medications and the delegation from medical to nursing through standing orders).
(3) State Pharmacy Law (defines the terms of prescribing, dispensing and administering of medication and who is legally authorized to do so).
(4) DEA, Controlled Substances Act of 1970 and Executive Order 12564, Drug-Free Federal Workplace, (regulations and laws for managing controlled substances).
(5) Copies of the laws and regulations listed above should be available in the Center Health clinic.
b. Security and Storage
Proper storage and security of medications in NASA Occupational Health clinics are a key risk-management function that ensures their integrity and prevents their deterioration.
Guidelines for appropriate storage of medications include the following:
(1) Lock medications in secured areas to avoid unauthorized access.
(2) Store in a secure area not readily accessible to employees, contractors or visitors.
(3) Store in area of constant supervision or surveillance.
(4) Organize and store for easy retrieval.
(a) Segregate by type, e.g., topical, oral, intravenous, to minimize the risk of medication errors.
(b) Store by therapeutic class rather than alphabetically to minimize the potential for medication error.
(5) Inspect routinely for expired or deteriorated medication and check that medications are in their designated location.
(6) Store according to the manufacturer's recommendations.
(a) Store in manner that addresses sanitation, temperature, light, moisture, ventilation, and segregation issues to maintain integrity.
(b) Store in a refrigerator, if needed, with adequate storage space.
(c) Store in refrigerator dedicated for medication use only (e.g., should not contain food or biological samples).
(d) Monitor temperature of the refrigerator daily.
(e) Ensure a system to alert of a power loss to the refrigerator (e.g., such as alarm or clock set on the same circuit).
(f) Monitor storage and security process by periodic random audits.
c. Medication Administration
The medication needs of any clinic must be determined based on the scope of care provided, demographics of the workforce, and nature of operative or potential risks in the workplace. There must be adequate staff qualified, competent, authorized to give medications, and meet State laws.
d. Considerations for the Workplace
There are a number of workplace considerations when administering medications to workers. Many medications contain substances or compounds, such as alcohol or antihistamines, that may cause drowsiness, impair performance, or potentially cause serious work-related injuries. Medication side effects, such as drowsiness, can impair performance including the operation of heavy equipment, driving a motor vehicle, or interfering with flight operations.
(1) Maintain a current copy of Physicians' Desk Reference and Physicians' Desk Reference for Nonprescription Drugs.
(2) Ensure familiarity of medications' desired and undesired effects, side and toxic effects, potential interactions, and the potential allergic reactions.
(3) Assess the potential effects of the medication on employees' ability to perform their jobs safely.
(4) Review current or past health conditions and prescription or nonprescription drugs including herbal remedies with the employee.
(5) Consider implications to other medical conditions such as hypertension, glaucoma, or diabetes.
(6) Provide information about the medication, and potential medication interactions.
(7) Promote self-care and consumer awareness about different products.
e. Standing Orders
Standing orders for all medications available in the NASA Occupational Health clinics provides a standardized approach to providing safe, quality care to employees. Such guidelines and orders establish the standards of care used for peer review and audit purposes.
(1) Establish standing orders for both prescription medicationand Over-The-Counter (OTC) medications available in the clinic.
(2) The drug, dosage, indications, contraindications, and adverse reactions should be included.
(3) The Medical Director and Chief Nurse must write, date, and sign the order.
(4) Standing orders must be reviewed, re-signed, and dated annually.
f. Medication Administration Issues
Prior to prescribing a medication, the healthcare provider must evaluate the patient. The findings of the history and physical examination and the treatment plan must be documented in the employee's medical record. The healthcare provider must discuss the benefits versus the risks of treatment and obtain verbal or written consent from the employee for treatment. The employee's condition must be monitored until the health issue is resolved or employee's care is transferred to another healthcare provider.
(1) Ensure staff competency through orientation, continuing education, and training.
(2) Provide staff education before new drug(s) added to the formulary.
(3) Ensure familiarity of the medication indications, dosages, side effects, and interactions with other medications.
(a) Review food or herbals, precautions to be taken, and any known allergies the employee may have.
(b) Maintain antidotes on hand in the event of adverse reactions to the medications.
(4) Identify the person, medication, time, dosage, route and technique prior to administering.
(a) Employees should be asked about any known allergies.
(b) Check the expiration date on the medication package.
(5) Provide written patient information sheets.
(6) Accept verbal orders in emergency situations; document as soon as possible.
(7) Verify verbal orders over the telephone after they are written and repeated for the physician, and countersign as soon as possible.
(8) Maintain a sample drugs system that allows for quick retrieval in the event of a recall.
(9) Utilize manufacturer's prefilled syringes with retractable needle.
(10) Utilize eye drop containers on one employee only.
(11) Report medication errors immediately.
(a) Discuss openly and share lessons learned.
(b) Report medication errors to risk management and quality improvement.
(c) Conduct root cause analysis for all medication errors.
(12) Target improvement in medication administration process proactively.
g. Medical Record Documentation
Documentation of the initial evaluation and subsequent visits must be made in the medical record. The format recommended is the Subjective, Objective, Assessment and Plan (SOAP). Medication(s) administered must be included in the documentation. Additionally, a list of all medications stocked in clinics must be maintained for prescription, emergency, and controlled substances. Documentation of medications includes the following:
(1) Identify employee allergies or positively note their absence where easily visible on the medical record.
(2) Document on summary sheets significant health conditions, current medications, and allergies.
(3) Record dosage, frequency, and amount in employee health record.
(4) Record patient instructions and discussion of adverse reactions.
(5) Record lot number for sample medications given.
h. Over-the-counter Medications
The stocking of OTC medications in the Center OH clinics must be based on the needs of the Center population and the scope of occupational health services provided.
(1) Maintain a current copy of the Physicians' Desk Reference for Nonprescription Drug.
(2) Adhere to standing orders when providing treating.
(3) Ensure adequate packaging and appropriate instructions on the package labeling.
(4) Provide in manufacturer's original unopened container without any type of medical or pharmaceutical intervention.
(5) Utilize unit dose packing to minimize problems associated with repackaging and cross contamination.
i. Prescription Medications
a. The use of prescription medications at Center Occupational Health clinics is based on need, staffing, and the defined scope of practice. The physician responsible for ordering the prescription medications must comply with Federal and State laws. Since State laws differ, it is critical they be reviewed for compliance with practices within the clinic setting. For example, State law determines whether prescription medications can be administered per standing orders and whether a physician must be present during the administration of prescription medications. Some States require a dispensing license.
(1) Maintain a current copy of the Physician's Desk Reference.
(2) Maintain an inventory for all prescription medications including sample drugs.
(3) Follow the standing orders when administering medications.
(4) Ensure familiarity with the drugs stocked, including the indications, contraindications, precautions, dosages, side effects, and the potential adverse reactions.
(5) Provide employee a medication information sheet.
(6) Document all medications received, administered, or discarded.
(7) Post a sign regarding generic versus brand name drugs in the clinic, if required by State laws.
(8) Prepare for emergencies since the potential exists for adverse reactions.
j. Cardiac Emergency Drugs
The cardiac cart must be stocked with emergency drugs recommended by the American Heart Association (AHA) treatment protocols.
(1) Maintain a list of drugs on the cardiac cart and postand posted along with treatment protocols.
(2) Maintain an inventory of the drugs and replace drugs prior to the expiration date.
(3) Maintain manufacturer's documentation if drug expiration date is extended due to shortages.
(4) Locate the cart in an area accessible in the event of an emergency.
(5) Keep cart locked or have an integrity tag in place at all times.
(a) Record lock numbers, when used.
(b) Document the reason locks or integrity tags are replaced.
(6) Make cart readily accessible to all Advanced Cardiac Life Support (ACLS) personnel.
k. Controlled Substances
Based on a needs and risk assessment, there may be a need to stock a limited amount of controlled substances in the clinic. It is mandatory to comply with State and Federal laws and regulations. A Drug Enforcement Administration (DEA) certificate is required when dispensing or administering controlled substances. A physician must be licensed in the State of practice to obtain the DEA certificate which must be posted with the clinic practice address on it.
(1) Maintain an inventory log showing the drugs received, administered, or disposed.
(a) Include the name and address of the physician, the DEA registration number, date, and time of inventory.
(b) Conduct the inventory with a witness; both physician and witness must sign the inventory.
(c) Retain the inventory and transaction log for a period of 2 years.
(d) Provide the inventory for inspection to the Board of Medical Examiners when requested.
(2) Document each dispensing transaction.
(3) Include the name of the employee, their social security number, name of the drug, quantity prescribed, dosage, date dispensed, the physician prescribing, and the signature of the healthcare provider dispensing the inventory drug.
(4) Store controlled substances in a securely locked, preferably double locked, and substantially constructed cabinet or safe.
(5) Maintain a minimum amount of stock in the inventory.
(6) Restrict access to drugs to key healthcare personnel only.
(7) Report any missing drugs to the DEA, notify the police, Center Medical Director,
(8) Risk Manager, Center Director, Security, and the NASA OHP Medical Director.
l. Immunizations
As a service of the NASA OHP, immunizations are offered to employees to protect them from vaccine-preventable disease. The need for vaccines is based on the employee's occupation, lifestyle, and health status. Assessment of an individual's risk for vaccine- preventable communicable disease must be made during health maintenance examinations, medical surveillance examinations, and in preparation for international travel. Vaccines, such as influenza, may be provided as part of health promotional campaigns. Prior to the administration of vaccines, clinical guidelines and standing orders must be in place.
(1) Complete a review of immunization history.
(2) Document the immunization history, if not previously documented, and employee's current health status.
(3) Complete an assessment of overall health status including any possible allergies, existing pregnancy and any immuno-compromised status.
(4) Determine vaccines needed.
(a) Review the vaccine indications, contraindications, precautions, dosages, side effects, and potential adverse reactions.
(b) Ensure completion of informed consent.
(c) Administer vaccine per standing orders utilizing proper aseptic technique.
(d) Document vaccine given, manufacturer, lot number, location of injection site, date and time given, any reactions, and due date of next vaccine.
(5) Provide a copy of the Centers for Disease Control (CDC) Vaccine Information Statement (VIS) for the vaccine(s) administered.
(6) Prepare for emergencies since the potential exists for adverse reactions.
(a) Report any adverse reaction to a vaccine to the CDC's Vaccine Adverse Event Reporting System (VAERS).
(b) Send a copy of the CDC VAERS form to the NASA OHP Medical Director.
(7) Store and dispose of vaccines according to the manufacturer's recommendations.
m. Allergy Injections
The NASA OHP supports the administration of allergy injections as a convenience for employees. Individual clinic decisions to offer this level of service should be based on the service volume, adequate staffing, emergency readiness and willingness to accept responsibility, and accountability for potential adverse reactions.
(1) Maintain written policy, procedures and standing orders.
(2) Require a written physician's order for allergy injection administration requests.
(3) Require order to contain employee's name, physician's name, address, and procedures to follow if dosage or timing is missed.
(4) Require employees to receive the first two allergy injections from treating physician.
(5) Require any employee with history of serious or anaphylactic reaction to see treating physician.
(6) Require a signed an informed consent form prior to beginning injection series.
(7) Store sera in a refrigerator containing only medications.
(8) Utilize safe and aseptic practices when administering injections.
(9) Require employee to remain in the clinic for 20 to 30 minutes for observation.
(10) Require employee resuming allergy injections after a 4-month lapse to receive the first two injections from treating physician.
(11) Prepare for emergencies since the potential exists for adverse reactions.
See also 3.1.4 Handling of Narcotics and Other Regulated Drugs.
n. Emergency Readiness
Emergency readiness is absolutely essential when offering allergy injections, vaccines, and other medications.
(1) Require all staff (e.g. receptionist, and others) to be Basic Life Support (BLS) certified.
(2) Require all nurses and staff administering the vaccines or injections to be ACLS certified.
(3) Require a physician, certified in ACLS, to be present in clinic area when injections or vaccines are administered and during postinjection observation period.
(4) Ensure that emergency procedures are in place and emergency equipment and medications readily available.
(a) Maintain oxygen and epinephrine available in the treatment administration room.
(b) Post emergency telephone numbers near the telephone.
3.1.2.4 References
a. Accreditation Association for Ambulatory Health Care.
b. Executive Order 12564, Drug-Free Federal Workforce.
c. Joint Commission on Accreditation of Healthcare Organizations
d. NASA NPD Control of Narcotics and Other-Regulated Drugs.
e. Physicians Desk Reference 2000.
f. Physicians Desk Reference For Nonprescription Drugs 2000.
a. Physicians Manual: An Informational Outline of the Controlled Substance of 1970.
b. Randolph, Susan A. (1996). Medication Management in the Workplace. AAAOHN Journal, 44(10), 508-512.
c. Rogers, B. Randolph, S. A. and Mastroianni, K. (1996). Occupational Health Nursing Guidelines For Primary Clinical Conditions. Beverly Farms, MA: OEM Press.
j. The Institute for Safe Medication Practices.
3.1.2.5 Flow Diagram
The flow diagram for this process is shown in Figure 3.1.2 at the end of this section.
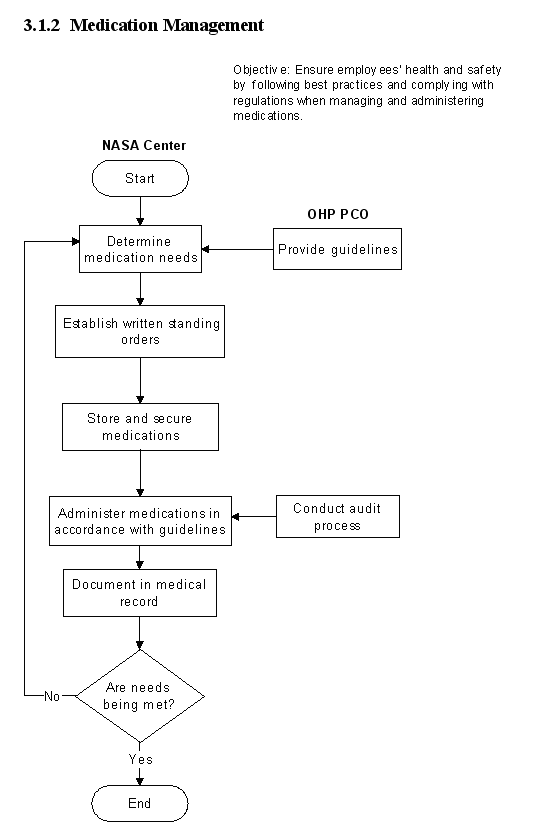
3.1.3 Diagnosis and Treatment of Occupational Illness or Injury
3.1.3.1 Introduction
Work-related injury and illness are a leading cause of morbidity and mortality in the labor force and are responsible for decreased productivity and substantial financial costs. Timely diagnosis and treatment of occupational injuries and illnesses are extremely important because they provide an opportunity not only to help the affected employee but also to prevent the recurrence of a similar problem in their coworkers and those in similar jobs.
3.1.3.2 Responsibilities
a. The Medical Director at each NASA Center is responsible for accurate diagnosis and timely treatment of all occupational injuries and illnesses in employees. As the "owner" of this process, this physician is ultimately responsible for initial care, followup, and recovery of the affected employee. Based upon patient history, timeline, and physical findings, the Medical Director will confirm or describe any inconsistencies with a work-related injury or illness. The Medical Director is also responsible for reporting all work-related injuries and illnesses to Center personnel responsible for OSHA recordkeeping.
b. All deaths, work-related or not, will immediately be reported to OHP for forwarding to OCHMO.
c. Any developing trends in diagnosis will be reported to OHP.
3.1.3.3. Process Description
a. The occupational health history is fundamental to the assessment of work-related , health problems. Addi , tio nal , ly, a total employme nt history and general health history, including a review of systems and determination of any preexisting conditions are important in achieving an accurate medical diagnosis.
b. The next step should be a complete physical examination with a detailed specific organ or system examination. Laboratory and radiological testing can also be used to complement the history and physical examination and to aid in the diagnosis.
c. Finally, an assessment of the work place by medical and/or safety personnel to enforcesure injury prevention and implementation of approved reasonable accommodation is crucial. All occupational health practitioners should become familiar with employees' work and the environment in which they work. This often necessitates a visit to the workplace and close workings with other areas such as Safety, Workers' Compensation (WC), and Employee Assistance Program (EAP) personnel.
3.1.3.4 References
3.1.3.5 Flow Diagram
The flow diagram for this process is shown in Figure 3.1.3 at the end of this section.

3.1.4 Handling of Narcotics and Other Regulated Drugs
3.1.4.1 Introduction
NASA Occupational Health clinics that store narcotics and other regulated drugs need to have and follow policy/procedures that ensure current regulatory compliance for the handling and storage of such drugs. Regardless of the location of the drugs, whether in medicine cabinets, crash carts or training kits, storage and security criteria must be met. Regulated drugs and medications may be maintained in a variety of locations, based on patient treatment areas and the physical facilities. Any unusual occurrences must be reported immediately to risk management.
3.1.4.2 Responsibilities
a. The Medical Director at each NASA Occupational Health clinic is functionally responsible for compliance with policy/procedures and applicable Federal and State regulations. Additionally all medical personnel are responsible for complying with the policy and regulations.
b. The NASA Centers are responsible for establishing guidelines for the handling of narcotics and other regulated drugs consistent with NASA policies.
c. The Center Drug Inventory Officer shall be a NASA employee, who is neither assigned to the medical staff of the Center's health clinic, nor is the supervisor of the Center's health clinic. The Center Drug Inventory Officer is responsible for maintaining accurate records for controlled drugs, and validating inventory and transaction records at prescribed intervals.
d. The NASA OHP conducts periodic auditing of NASA Centers' drug policies and procedures.
3.1.4.3 Process Description
Elements of the process include--
a. An assessment of the patient population and potential health risks is the basis for decisions on the appropriate drugs to stock in the clinic setting.
b. A physician is designated responsible for managing the drug program
c. The Medical Director is responsible to for establishing the process and procedures for managing the drugs.
d. The Medical Director provides education to the healthcare providers responsible for ordering and dispensing of medications.
e. The Medical Director maintains documentation on all drugs procured, dispensed, discarded, or destroyed.
f. The Center Drug Inventory Officer is responsible for establishing, maintaining and auditing the inventory of controlled drugs on a periodic basis.
g. The Center Drug Inventory Officer is responsible for all expired drugs being disposed of according to State and Federal regulations.
h. The Center Drug Inventory Officer is responsible for any unusual occurrences, such as missing drugs or unusual patterns of ordering and for reporting to risk management.
3.1.4.4 References
a. Controlled Substances Act of 1970 (84 Stat. 1242 U.S.C. 801, et seq.1970 Acts Section 704 of Pub.L. 91-513, 84 Stat. 1242, 21 U.S.C. 801 et seq.).
b . See Section 3.1.2, Medication Management, and Appendix D, Medication Management Checklist.
3.1.4.5 Flow Diagram
The flow diagram for this process is shown in Figure 3.1.4 at the end of this section.
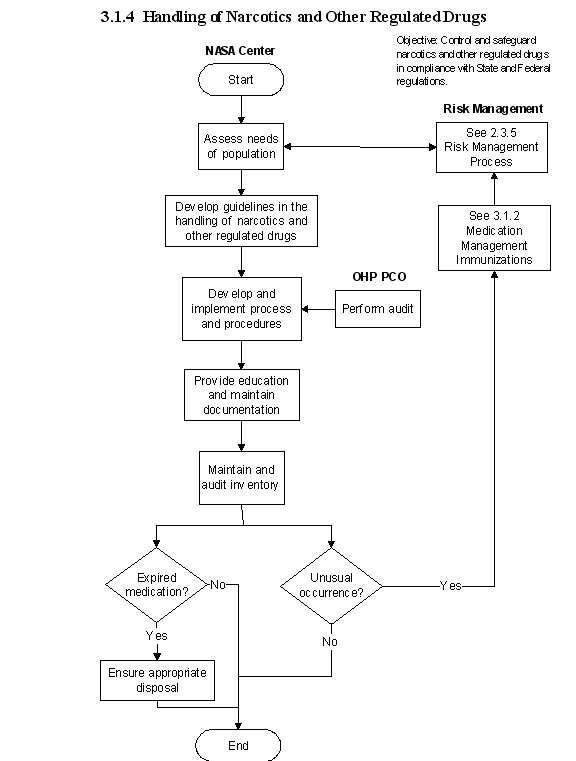
3.1.5 Medical Review Officer Drug Free Workplace Program Support
3.1.5.1 Introduction
On September 15, 1988, President Reagan signed Executive Order (EO) 12564, Drug-Free Federal Workplace, which established the goal of a drug-free workplace and made it a condition of employment for all Federal employees to refrain from using illegal drugs on or off duty. The NPR 3792.1, Plan For A Drug-Free Workplace, was developed to implement NASA policy.
3.1.5.2 Responsibilities
a. The DPM ensures that all processes and procedures documented in the NPR are uniformly implemented and conducted throughout the Agency.
b. Reserved.
c. Reserved.
d. A Screening Test Technician (STT) who has training in screening test techniques, may be designated by the Center DPC only after the screening test has been approved for Center use by the NASA DPM and the Agency MRO.
e. The Center Director designates a Drug Program Coordinator (DPC), who is responsible for implementing, directing, administering, and managing the program at the Center and notifies the employee of test results confirmed by the Center MRO.
f. The Center-trained and-certified MRO receives test results directly from the certifying drug test laboratory, confirms positive test results, and reviews them with the Agency MRO prior to notifying the Center. The validity of positive and negative test results is confirmed administratively. Normally, the Center Medical Director is also the Center MRO. If another person is designated as the Center MRO, the Agency MRO must approve the person's credentials.
3.1.5.3 Process Description
a. The AgencyNASA DPM determines the tests needed for the program and which job categories and circumstances in NASA will require drug testing.
b. The Center DPC ensures that drug test sampling at the Center conforms to NASA policies and that collectors of the specimen (Center MRO, if needed) are properly trained to perform their responsibilities.
c. Collections are obtained respectfully, in an evidential manner with Chain of Custody (COC) and confidentiality properly maintained. All positive test results or changes of findings are reviewed by the Agency MRO to confirm validity of findings.
d. Only approved forms and laboratories are utilized. The Agency MRO does not review approved screening tests since the tests only serve to decide if a drug test is indicated. Only test results are reviewed by the MRO.
e. If a nonevidential test is performed, the test must have prior approval for use at a Center by the AgencyNASA DPM and MRO.
f. Audits, maintaining employee confidentiality of MRO test results, and COC completeness records will be available to the Agency MRO and NASA DPM if necessary for Quality Control purposes.
3.1.5.4 References
a. NPR 3792.1, Plan For A Drug-Free Workplace.
b. DG-01NASA, Desk Guide on the Drug-Free Workplace Program, October 1996.
c. 10 CFR Part 26, Nuclear Regulatory Commission regulations governing Fitness for Duty Programs.
d. 10 CFR Part 121, Federal Aviation Administration/Department of Transportation regulations for Conducting Anti-drug and Alcohol Abuse Prevention Programs for Safety-Sensitive Employees in the Aviation Industry.
e. 49 CFR Part 40, Office of the Secretary of Transportation regulations governing Procedures for Transportation Workplace Drug Testing Programs.
f. 59 FR 29908, Mandatory Guidelines for Federal Workplace Drug Testing Programs, Federal Register, June 9, 1994.
g. EO 12564, Drug Free Federal Workplace, Sept. 15, 1988.
h. SAMHSA, Substance Abuse and Mental Health Services Administration, Current List of Laboratories Which Meet Minimum Standards to Engage in Urine Drug Testing for Federal Agencies.
3.1.5.5 Flow Diagram
The flow diagram for this process is shown in Figure 3.1.5 at the end of this section.

3.1.6 Medical Review Officer Activities
3.1.6.1 Introduction
a. NPR 3792.1A, Plan For A Drug-Free Workplace, defines the MRO roles and responsibilities for NASA. In addition, both the NPR 3792.1A and 49 CFR Part 40, state that "Each Center's MRO must be a licensed physician with knowledge of substance abuse disorders and the appropriate medical training to interpret and evaluate all positive test results together with an individual's medical history and any other relevant biomedical information." The Center MRO may be a NASA employee or a contractor for the Agency.
b. Over the years, the accepted medical standard for qualification has become formal training and certification. The American Association of Medical Review Officers (AAMRO) provide both a training course and certification examinations. The Medical Review Officers' Certification Council (MROCC), is the organization that certifies MRO's. Several States (e.g., Oklahoma in 1994, Florida in 1996) have adopted laws mandating training and certification for MRO's.
c. Reserved.
3.1.6.2 Responsibilities
a. The Center MRO, who is trained and certified confirms positive test results and reviews them and any test result changes with the Agency MRO prior to notifying the Center. The validity of positive and negative test results is confirmed administratively. If another person is designated as the Center MRO, the Agency MRO must approve the person's credentials.
b. The Center Director designates a DPC, who is responsible for implementing, directing, administering, and managing the program at the Center and notifies the employee of test results confirmed by the Center MRO.
c. The NASA Agency MRO develops, implements, and evaluates EAP and Agencywide medical review functions, including Center MRO confirmation and negation of test results as it relates to drug and alcohol testing. The Agency MRO should have additional training as a BAT to qualify for reviewing questions related to EBT's.
d. The NASA Associate Administrator for Human Resources and Education ensures that NPR 3792.1A is implemented, establishes the processes and procedures to carry out the plan, and designates the NASA DPM.
e. The DPM ensures that all processes and procedures documented in the NPR are uniformly implemented and conducted throughout the Agency.
3.1.6.3 Process Description
a. In order for the Center MRO to confirm a result, the following procedures must be completed.
(1) The specimen of the individual selected under Center Drug Program procedures must have been collected properly.
(2) The Chain of Custody (COC) and security of the specimen must have been maintained.
(3) The laboratory testing the specimen must be properly certified.
(4) All test results (positive or negative) must be sent directly to the MRO in a confidential manner.
(5) The specimen must not be adulterated (including excessive dilution that would interfere with test result) or substituted.
(6) The individual with a positive test result must be given the opportunity to provide a medical reason for a false-positive test.
(7) A positive test result--whether the test remains positive or is negated as a positive test--must be reviewed with the Agency MRO.
b. Once a determination has been made that the test is a valid test, then the procedures shall be followed.
(1) The MRO-confirmed test result is transmitted to the Center DPC to determine if--
(a) Testing of a prior obtained split specimen is desired by the individual.
(b) If the individual meets program requirements to obtain a repeat sample if the test nonvalid.
(2) Reserved.
c. If the test is determined to be invalid because of failure to show, failure to provide an adequate collection sample, adulteration, excessive dilution, or failed COC, then the MRO contacts the DPC who determines whether a repeat collection and sample must be obtained under the rules of the program, and the next actions to be taken.
d. If a correctable flaw is found with the COC, the MRO will notify the Center DPC who will assist with obtaining a correction. If the flaw is noncorrectable or "Fatal," the "Test Not Performed" or "Test Cancelled" finding will be marked in Step 8 of the COC form and the DPC contacted.
e. A test may be determined to be positive without having communicated with the employee only under three circumstances.
(1) The employee expressly declines the opportunity to discuss the test. Written documentation of the time and date and statement should be made.
(2) Neither the MRO nor the DPC as the designated employer representative, after making all reasonable efforts, has been able to contact the employee within 14 days of the date on which the MRO receives the confirmed positive test result from the laboratory. DPC assistance should be asked, if no contact with the employee to discuss the results is made by the MRO after 3 working days.
(3) The DPC as the designated employer representative has successfully made and documented a contact with the employee and instructed the employee to contact the MRO, and more than five days have passed since the date the employee was successfully contacted by the DPC.
f. Reserved.
(1) Reserved.
(2) Reserved.
(3) Reserved.
(a) Reserved.
(i) Reserved.
(ii) Reserved.
(iii) Reserved.
(iv) Reserved.
(v) Researved.
(b) Reserved.
(i) Reserved.
(ii) Reserved.
(iii) Reserved.
(iv) Reserved.
g. If the MRO verifies the test as Positive, the MRO checks the Positive indication with appropriate remarks and reports the result to the employer. If the MRO verifies the test as Negative, the MRO checks the Negative indication and reports the result to the employer. If the laboratory also reports that the specimen was diluted, the MRO reports to the employer that the next time the donor is selected for a drug test, the employer may require the specimen to be collected under direct observation.
3.1.6.4 References
a. NPR 3792.1, Plan For A Drug-Free Workplace.
b. DG-01, NASA, Desk Guide on the Drug-Free Workplace Program, October 1996.
c. 10 CFR Part 26, Nuclear Regulatory Commission regulations governing Fitness for Duty Programs.
d. 10 CFR Part 121, Federal Aviation Administration/Department of Transportation regulations for Conducting Anti-drug and Alcohol Abuse Prevention Programs for Safety-Sensitive Employees in the Aviation Industry.
e. 49 CFR Part 40, Office of the Secretary of Transportation regulations governing Procedures for Transportation Workplace Drug Testing Programs.
f. 59 FR 29908, Mandatory Guidelines for Federal Workplace Drug Testing. Programs, Federal Register, June 9, 1994.
g. EO 12564, Drug Free Federal Workplace, Sept. 15, 1988.
h. SAMHSA, Substance Abuse and Mental Health Services Administration, Current List of Laboratories Which Meet Minimum Standards to Engage in Urine Drug Testing for Federal Agencies.
i. Memorandum, MRO Guidance for Interpreting Specimen Validity Test Results, Department of Health and Human Services, Sept 28, 1998, to Medical Review Officers (Certified and Applicant) from Director, Drug and Alcohol Policy and Compliance Office.
3.1.6.5 Flow Diagram
The flow diagram for this process is shown in Figure 3.1.6 at the end of this section.
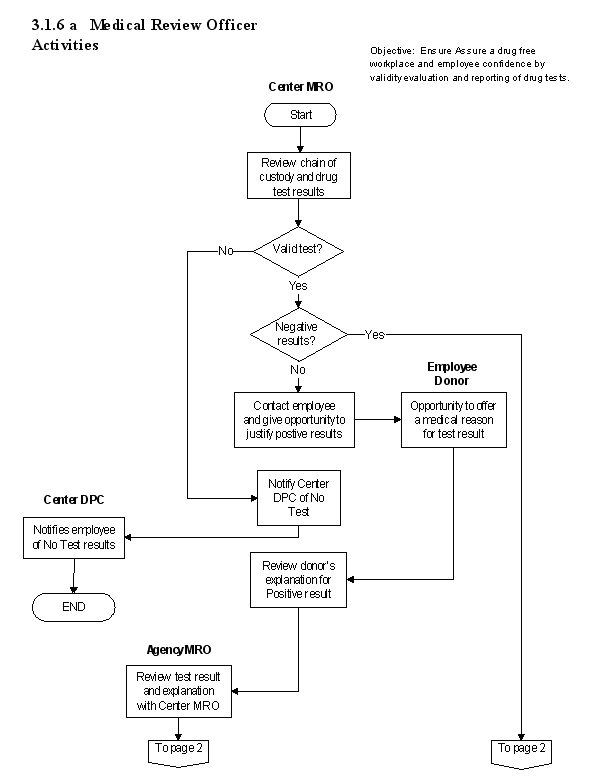
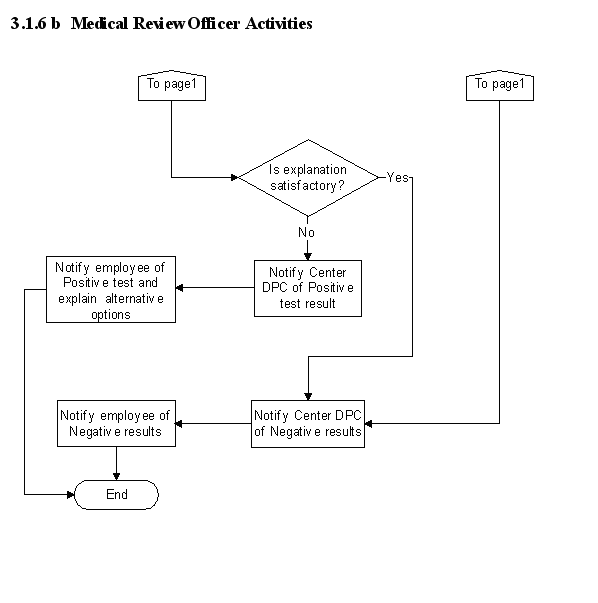
3.2.1 General Physical Examinations
NASA operations require use of a wide spectrum of physical examinations. The complexity and frequency of these examinations are also quite varied. Some are comprehensive while others may involve only evaluation of a single body system or function. Certain aspects of physical examinations are common to all; section 3.2.1 addresses these generically. Specific and tailored examinations are described in succeeding sections, accompanied by rationale and content. This chapter assembles the basics of most routine and specialty examinations performed at NASA Centers into one procedures chapter.
The expanded listing provided here gives an overview of the many types of physical examinations required in NASA Center operations. Appendix G provides a concise overview in matrix format.
a. General Physical Examinations 95
b. Preplacement Examinations 99
(1) Civil Service Personnel
(2) Pre-Employment Screening
c. Surveillance Examinations 101
(1) Workers with Specific Potentially Hazardous Exposures
(a) Arsenic
(b) Asbestos
(c) Benzene
(d) Beryllium
(e) Cadmium
(f) Chromium
(g) Ethylene Oxide
(h) Formaldehyde
(i) Lead
(j) Mercury
(k) Methylene Chloride
(l) Methylene Diisocyanate, Methylene Triisocyanate
(m) 4,4'-Methylene Bis (2-Chloroaniline) and 4,4'-Methylene Dianiline
(n) Phenylenediaminie
(o) Nickel
(p) Polychlorinated Biphenyls
(q) Thermal Ablative, Methylene Biphenyl Isocyanate
(r) Trichloroethylene
(s) Tungsten Carbide
(t) Noise (Hearing Conservation)
(u) Silica Dusts
(2) Workers in Potentially Hazardous Environments
(a) Chemical Laboratory Workers
(b) Hazardous Waste Workers
(c) Hearing Conservation
(d) Spray Painting
(e) Water and Sewage
d. Job Certification Examinations 115
(1) Air Traffic Controller
(2) Confined Space/Tank Entry
(3) Crane Operator/Ground/Floor/Remote Operated Cranes
(4) Crane Operator/High
(5) Diver
(6) Down Range/Shipboard and Remote Assignments
(7) Federal Aviation Administration Personnel
(8) Fire Fighter
(9) Food Handler
(10) Fuel Handler/Contingency Crew
(11) Hazardous Materials Emergency Response Team
(12) Heavy Ordinance
(13) High Crew/Spider
(14) Locomotive Engineer/Crawler-Transporter
(15) Motive (Heavy) Equipment Operators
(16) Multiple Passenger Vehicle Operator
(17) Department of Transportation Commercial Drivers License
(18) Motor Vehicle Certification
(19) Non-Crew Flying
(20) Self Contained Atmospheric Protective Ensemble (SCAPE)
(21) Security Personnel
(22) Solid Rocket Booster Retrieval
e. Special-Purpose Examinations 127
(1) Employment Situations
(a) Disability Retirement
(b) Fitness for Duty - General
(c) Fitness for Duty - Civil Service
(d) Fitness for Duty - Contractor
(e) Return to Work
(2) Visual Examinations
(a) General Examinations
(b) Console Color spectrum
(c) Dye Penetrant
(d) Microscopic Particle Counting
(e) Solderers and Welders
(3) Workplace Exposures
(a) Bloodborne Pathogens
(b) Laser Workers
(c) Primary Animal Contact
(d) Primary Crew Contact
(e) Ionizing Radiation Workers
(f) Respirator, Occupational (Non-SCAPE)
(g) Respirator, Other
(h) Tuberculosis Control
f. Health Maintenance Examinations 138
(a) Federal Employees Health Program
(b) NASA Executives
(c) Key Contractor Executives
3.2.1 General Physical Examinations
3.2.1.1 Introduction
a. Reserved.
b. Physical examinations are performed for several purposes. Local, state or federal regulations may direct physical examinations for certain categories of employees. The employer, employee, or an examining physician may also request examinations. They may also be a part of the employer's standard operating procedures. Regardless of the reason for initiating the examination, the purpose is to protect the health and well-being of employees and coworkers, and to ensure their optimal work performance.
c. Examinations are structured to determine if the employee can safely and adequately accomplish the essential functions of a job, to determine if performance of the job has produced any evidence of an effect on the employee's health, or to monitor the general health of the employee. A single examination may serve several purposes. Results of the examinations are communicated to the employee and the employer, and are made available to the employee's personal physician within the strict guidelines on confidentiality and Code of Ethical Conduct.
d. Regarding currency of an examination, if a physical examination has been conducted within the previous six months and has been duly recorded in the employee's health record, it may, at the discretion of the examining physician, be accepted in whole or in part as the requested medical examination. Complete examinations conducted more than 6 months previously may be utilized with appropriate supporting information and a signed interval note by the cognizant examining physician. A physical examination conducted for one purpose is valid for any other purpose within the prescribed validity period if that physical contains the proper data. If the examination is deficient in scope, only those tests and procedures needed to meet the additional requirements are performed. The results are recorded and the examining physician signs the appropriate approval.
e. Physical examinations are grouped into five categories: Preplacement, Surveillance, Job Certification, Special Purpose, and Health Maintenance.
3.2.1.2 Responsibilities
a. Managers and supervisors identify, with occupational medical, environmental health, and safety consultations, as appropriate, which jobs require any level of medical evaluation, if hazards exist to workers, job certification and employee medical surveillance are indicated. Special-purpose and health maintenance examinations will be required at the discretion of Senior Management according to corporate policy and other regulatory guidelines.
b. Reserved.
c. All disciplines required in health care delivery are responsible in their areas of expertise for accomplishing and interpreting medical evaluations.
d. Appeal, redress, second opinions, and challenged decisions will be handled at the lowest level of authority. The NASA Medical Policy Board holds final decision authority in contested situations.
3.2.1.3 Process Description
a. The following recommended intervals are given for those situations where there are no established frequency guidelines. These recommendations are not intended to take precedence over Center requirements.
|
Type |
Frequency |
|
Preplacement |
As required |
|
Surveillance |
Annually unless otherwise stated |
|
Job Certification |
Age-determined unless otherwise stated |
|
Health Maintenance |
Age-determined unless otherwise stated |
|
Special-Purpose |
As required |
|
Age |
Frequency |
|
Up to age 40 |
Every 5 years (ages 20, 25, 30, 35, 40) |
|
Age 40-50 years |
Every 2 years (ages 42, 44, 46, 48, 50) |
|
Over age 50 |
Annually |
b. A complete physical examination is to be performed in all preplacement and initial surveillance examinations, and in most job certification and health maintenance examinations.
c. Reserved.
d. Every complete physical examination includes (unless the employee declines) the following:
(1) Disrobing
(2) Head, Eyes, Ears, Nose and Throat (HEENT), including ophthalmoscopic
(3) Lymphatic
(a) Cervical
(b) Axillary
(c) Inguinal
(4) Pulses
(a) Carotid
(b) Radial
(c) Dorsalis pedis/posterior tibial
(5) Auscultation of heart, lungs, and abdomen.
(6) Abdominal palpation and inspection.
(7) Inguinal hernia examination.
(8) Scrotum and contents examination.
(9) Inspection of extremities.
(10) Rectal and prostate examination at age 40 and over are performed with the physical examination, unless the employee testifies to examination by a private physician within 1 year.
(11) Hemoccult offered in accordance with the American College of Surgeons (ACS) recommendations.
(12) Female breast examinations are offered.
(13) Females are advised regarding the best available current mammography recommendations and strongly encouraged to have appropriate studies.
(14) Pelvic examination and Papanicolaou (Pap) tests are offered to those female employees in the Federal Employees Health Program (FEHP), active duty military service, and the executive physical examination program. This examination may not be needed if the employee testifies to a normal examination by a private physician within the previous year.
(15) Additionally, documentation of the completed examination includes--
(a) Annotation to reflect positive or negative findings relevant to items raised by the history.
(b) Comments on all abnormal findings.
(c) Record of diagnosis.
(d) Record of recommendations.
3.2.1.4 References
a. Centers for Disease Control (CDC) "Blue Sheet" (Weekly summary of countries with infected areas of Quarantined Disease and Health Information for International Travel).
b. Federal Aviation Administration (FAA) Guide for Aviation Medical Examiners.
c. Federal Personnel Manual Supplement 339-31, Reviewing and Acting on Medical Certificates.
d. 29 CFR Part 1910.120, Occupational Safety and Health Administration, 29 regulations governing Hazardous Water Operation and Emergency Response.
e. Respirator Medical Evaluation Questionnaire.
f. Refer also to Appendix G for a detailed matrix of all examinations described in sections 3.2.2 through 3.2.6.
3.2.2 Preplacement Examinations
3.2.2.1 Introduction
Preplacement physical examinations are conducted to determine if an employee can safely and adequately perform the essential functions of a job. The essential functions of a job are identified by the employer and communicated to the examining physician. Preplacement examinations are performed before assignment to the job. (The standards for NASA and other civil service personnel are outlined in the Federal Personnel Manual Supplement 339-31, Reviewing and Acting on Medical Certificates. The physical requirements for the job are specified on the Standard Form (SF) 88, which is completed at the time of the examination.)
3.2.2.2 Reserved.
a. Reserved.
(1) Reserved.
(2) Reserved.
(a) Reserved.
(b) Reserved.
(c) Reserved.
(i) Reserved.
(ii) Reserved.
(iii) Reserved.
(iv) Reserved.
(3) Reserved.
b. Reserved.
(1) Reserved.
(a) Reserved.
(b) Reserved.
(2) The pre-employment examination includes the following:
(a) Occupational and medical history. (Utilize questionnaires where applicable.)
(i) Document past occupational exposures to chemical and physical hazards
(ii) Document past illnesses and chronic diseases such as atopy, asthma, lung diseases, renal and cardiovascular problems.
(b) Review symptoms, respiratory, high blood pressure.
(c) Identify employees who are vulnerable to particular substances.
(d) Document lifestyle habits such as smoking, alcohol and drug abuse.
(3) A complete physical examination is performed to identify conditions that may place the employee at risk. Examples are obesity, lack of conditioning, respiratory problems such as chronic obstructive pulmonary disease, facial abnormalities, and orthopedic problems such as severe arthritis, missing digits.
(4) Laboratory data.
(a) Sequential Multiple Analyzer Computer (SMAC) blood chemistry profile, Complete Blood Count (CBC), Urinalysis (U/A), Pulmonary Function Test (PFT), Electrocardiogram (ECG), vital signs, visual testing, audiometric testing, Chest X-ray (CXR).
(b) Pertinent baseline biomonitoring as appropriate, e.g., Blood Lead Level (BLL) and Zinc Protoporphyrins (ZPP) for lead workers; Cadmium in Blood (CdB), Cadmium in Urine (CdU) and Beta-2 Microglobulin in urine (B2-M) for cadmium workers; Polychlorinated Biphenyl (PCB) levels for PCB workers; and acetylcholinesterase levels for pesticide workers.
3.2.3 Surveillance Examinations
3.2.3.1 Introduction
Identification of workers needing specific surveillance examinations is the responsibility of the employer. The examination may be for only one chemical exposure or a category of exposures. The extent of the "hands-on" examination, laboratory and special procedure examinations required for each category of surveillance physical is specified in writing. If the examinations are not performed onsite, the OHP Medical Director reviews the results before clearance is issued to work in a hazardous environment.
3.2.3.2 Process Description
This section provides guidance for treating workers with specific potentially hazardous exposures.
a. Arsenic
(1) Reference: OSHA 29 CFR 1910.1018
(2) Frequency:
(a) Preplacement for all employees who are or may be exposed to arsenic at or above the action level 30 or more days per year.
(b) Semiannually for employees 45 years old or older or 10 or more years of exposure over the action level.
(c) Annually for all other covered employees.
(d) Ad hoc examination if for any reason the employee develops signs or symptoms usually associated with exposure to inorganic arsenic.
(e) Termination if no examination has occurred within the past 6 months.
b. Asbestos
(1) Reference: OSHA 29 Part CFR 1910.1001 and 29 CFR Part 1926.1101.
(2) Frequency: Preplacement, annually and termination for asbestos workers.
(3) Medical evaluations and physical examinations are offered to employees exposed or potentially exposed to asbestos fibers in accordance with regulations listed above.
(4) Evaluation of asbestos exposure status consists of the following:
(a) Completion of the Occupational Health Questionnaire for Asbestos Workers (available in Appendix D, parts 1 and 2 of the OSHA Asbestos Standard) to include a detailed occupational history with special emphasis on exposure to dusts, fumes, fibers, gases, and any other respirable materials or compounds.
(b) History of respiratory system disease of any nature, e.g., infections, allergies, congenital abnormalities.
(c) Smoking history.
(d) History of other household members exposed to asbestos fibers.
(5) Incidentally exposed employees are offered a complete baseline examination, but no periodic examination is scheduled. (Incidentally exposed are those people who may have been exposed in excess of the action level on a one-time basis. EH personnel can assist to validate.)
(6) Civil service personnel requiring asbestos evaluation and/or surveillance may have these procedures combined with their FEHP examination.
(7) All findings, negative as well as positive, are noted in the medical record. The latter are identified and retained in accordance with 29 CFR 1910 asbestos regulations.
(8) Current CXR surveillance schedule is as follows:
(a) Baseline/preplacement Posterior-Anterior (PA) chest.
(b) Periodic PA chest schedule--
(1) 1-10 years since first exposure: CXR every 5 years.
(2) 10+ years since first exposure:
(3) Age 35: CXR every 5 years.
(4) Age 35-45: CXR every 2 years.
(5) Age 45+: CXR annually.
(6) Termination CXR in accordance with the periodic schedule.
(c) Only asbestos workers who have been exposed to airborne asbestos in excess of the action level or the excursion limit are medically monitored.
c. Benzene
(1) Reference: OSHA 29 CFR Part 1910.1028.
(2) Frequency: Preplacement, annually and ad hoc for employees exposed to airborne benzene at or above the action level, TWA, or STEL as applicable to the time spent working at these levels.
d. Beryllium
(1) Reference: OSHA 29 CFR Part 1910.1000.
The International Agency for Research on Cancer concluded in 1993 that "there is sufficient evidence in humans for carcinogenicity of beryllium and beryllium compounds."
(2) Frequency: Preplacement and annually for employees exposed at or above an action level.
e. Cadmium
(1) Reference: OSHA 29 CFR Parts 1910.1027 and 1926.1127.
(2) Frequency: A complete initial medical examination occurs within 30 days after the initial medical examination includes biological and medical monitoring. Each request for cadmium surveillance physical examination is validated by EH personnel. Medical surveillance is required for any employee who is, or may be, exposed at or above the airborne action level for 30 days or more per year. The frequency of monitoring initial assignment to a job with exposure to cadmium above the airborne action level depends upon the biomonitoring category as defined in the following sections:
(a) Biological monitoring includes SMAC, CBC, U/A, CdU, CdB, and B2-M. Biological monitoring occurs at least annually.
(b) Medical monitoring occurs with the initial examination at 12 months and at least every 2 years thereafter. The medical monitoring examination is performed by an OHP physician, or a health care professional designated by the physician, and includes the following in addition to the biologic monitoring:
(i) Baseline CXR (physician determines CXR frequency on an as need basis after baseline).
(ii) Detailed history, to include medical and work history, smoking status, reproductive history, nephrotoxic medication usage, any history of renal, cardiovascular, respiratory, hematopoietic, and/or musculoskeletal dysfunction.
(iii) Pulmonary function testing.
(iv) Physical examination to include prostate palpation for all males over 40 years of age, blood pressure, and Prostate Specific Antigen (PSA) testing for males over age 50.
(3) A medical examination is performed as soon as possible whenever an employee demonstrates difficulty in breathing during a respirator fit test, or during use of a respirator.
(4) The exit cadmium physical examination includes a CXR.
(5) For each medical examination, a physician-signed written medical opinion is sent to the employer. The employer is required to forward these data to the employee within 2 weeks of receipt. The written medical opinion includes the following:
(a) Any diagnosis related to cadmium exposure.
(b) Any detected health risks from cadmium exposure.
(c) Results of biological monitoring.
(d) Recommended removal or duty limitations.
(e) A statement that the physician has clearly and carefully explained to the employee the results of the medical examination, including all the biological monitoring results and any medical conditions related to cadmium exposure that requires further evaluation or treatment, and any limitations on the employee's diet or use of medications. The medical opinion does not include any findings or diagnoses unrelated to occupational exposure to cadmium.
(6) The biological action levels and biomonitoring categories will be dictated by current OSHA standards.
(7) All medical removals occur with the concurrence of the OHP Medical Director. Medical removal is at the discretion of the physician when exposure is between normal and "high triggers." Above the "high triggers," mandatory removal occurs. The employer is responsible for removing the employee from exposure to cadmium above the airborne action level as soon as possible after written notification from the examining physician has been received. The employee has certain Medical Removal Protection (MRP) rights as outlined by OSHA regulations.
(8) Records Retention: Records are retained for employment period plus 30 years.
f. Chromium
(1) Reference: OSHA 29 CFR Part 1910.1000
(2) While there are no OSHA, NASA, or State requirements for a chromium or chromic acid medical surveillance examination, special situations may exist when periodic surveillance examinations are advisable.
g. Ethylene Oxide
(1) Reference: OSHA 29 CFR Part 1910.1047
(2) Frequency: Preplacement, annually, and ad hoc when specified ethylene oxide worker is exposed at or above the action level.
h. Formaldehyde
(1) Reference: OSHA 29 CFR Part 1910.1048
(2) Frequency: Preplacement, annually, and ad hoc for employees exposed at or above the action level or exceeding the STEL.
i. Lead
(1) Reference: OSHA 29 CFR Parts 1910.1025 and 1926.62
(2) A medical surveillance program is provided by NASA for employees who are or may be exposed to an airborne concentration of lead at or above an OSHA action level. The medical surveillance program is under the direction of the OHP Medical Director and complies with the requirements established in the OSHA Lead Standards referenced above.
(3) NASA is responsible for identifying employees who may require enrollment in medical surveillance program. This information is provided to OHP medical personnel via the appropriate form notification. The employee is enrolled in the medical surveillance program, and the appropriate EH personnel determine if the employee meets the OSHA requirements for continued enrollment. The medical surveillance program includes biological monitoring and medical examination or consultation.
(4) Biologic monitoring consists of blood sampling for BLL and ZPP. The frequency of biologic monitoring is every 2 months for the first 6 months and every 6 months thereafter.
(5) A complete physical examination is performed as the initial preplacement examination and at least every 2 years. This frequency may be more often than 2 years, if deemed necessary by the examining physician.
(6) NASA makes an initial medical surveillance examination available to employees who are occupationally exposed on any day to an airborne lead concentration at or above the OSHA action level. This initial medical surveillance includes blood sampling for BLL and ZPP. The proper OSHA criteria have to be met, as determined by the appropriate EH personnel.
(7) The BLL action level will be dictated by current OSHA standards.
(8) OHP medical personnel forward all BLL values to NASA. NASA is required to provide a copy of the employee's BLL to the employee within 5 working days after the employer's receipt of the BLL results.
(9) A medical examination occurs if the BLL exceeds the action level. EH personnel are notified by OHP medical personnel to begin an evaluation of the employee's work practices and other aspects of lead exposure, if the BLL action level is met or exceeded. The OHP Medical Director or a physician designee performs the medical evaluation within a 2-week period after NASA receives notification of the elevated BLL. The BLL is repeated at the time of the medical evaluation. A medical examination also occurs as soon as possible on the following:
(a) After an employee develops or complains of signs and symptoms of lead intoxication,
(b) After an employee desires medical advice concerning the effects of current or past exposure to lead on the employee's ability to procreate a child, and
(c) After an employee, who is enrolled in the medical surveillance program, has demonstrated difficulty in breathing during a respirator fit test or during use, or
(d) After it is known that the employee is pregnant.
Any time employees have a medical examination mandated for any of the above reasons, they are notified by NASA of their guaranteed right to seek a second medical opinion from another physician of the their choice, regarding the occupational exposure to lead. Any discrepancy or dispute between the second physician and the initial evaluating physician is settled in consultations/negotiations or by a third physician.
(10) The medical examination contains a detailed work history, medical history, recording of possible past lead exposures, personal habits (smoking and hygiene), past gastroenterological, renal, cardiovascular, reproductive, or neurological problems. A physical examination is performed with attention to the teeth, gums, hematologic, gastrointestinal, renal, cardiovascular, pulmonary, and neurological status. Blood pressure, BLL, ZPP, and laboratory profile (to include hemoglobin, hematocrit, red cell indices, peripheral smear morphology, blood urea nitrogen/Cr, U/A with micro) are required. If requested by the employee, pregnancy testing and laboratory evaluation of male fertility is performed.
(11) Removal (MRP) of an employee from a worksite where lead exposure can occur at or above the OSHA airborne concentration of lead action level is mandatory if the BLL exceeds OSHA removal limits. NASA is responsible for removing the employee from lead exposure as soon as possible after the notification from OHP medical personnel is received. Once removed from a lead work environment due to an elevated BLL, the employee is permitted to return to work only after clearance is received from the OHP physician. BLL values are determined at least every month until it is determined that the employee can safely return to work. Clearance is given when two consecutive BLL values are at or below established levels and return to work is not medically contraindicated.
(12) The OHP physician may recommend removal of any employee from a worksite where the airborne concentration of lead exceeds the OSHA action level. This is done if a medical condition detecte in the employee, or if there is concern that a medical finding, determination, or opinion, would place the employee at increased risk of material impairment to health from exposure to lead.
(13) For each medical examination that requires employee removal from any occupational exposure to lead above the OSHA action level, a written statement is created by the examining physician and provided to the employee in accordance with the Privacy Act. The physician's opinion statement contains at least the following:
(a) Any detected medical condition that would place the employee at increased risk of material impairment of the employee's health from exposure to lead.
(b) Any recommended special protective measures or limitations to be placed upon the employee's exposure to lead.
(c) Any recommended limitation upon the employee's use of respirators, including a determination of whether the employee can wear a powered air purifying respirator or if a physician determines that the employee cannot wear a negative pressure respirator.
(d) The results of the BLL determination.
(14) The written opinion does not reveal any findings or diagnoses unrelated to an employee's occupational exposure to lead. However, the physician, either verbally or in writing, advises the employee of any medical condition (and properly records this advice in the medical chart), occupational or nonoccupational, which dictates further medical examination or treatment.
(15) Employees who are determined not to be medically qualified for work according to lead exposure requirements are to have a medical opinion statement sent directly to them in accordance with the Privacy Act. OHP medical personnel also inform the employer of the employee's status as appropriate for the circumstances.
j. Mercury
(1) Reference: Agency for Toxic Substances and Disease Registry (ASTDR), 1992
(2) Frequency: Complete preplacement examination, with annual laboratory screening. Complete physical examination at least every 2 years.
(3) Laboratory: SMAC, CBC, U/A, visual testing, PFT, 24-hour urine mercury.
k. Methylene Chloride
(1) Reference: OSHA 29 CFR Part 1910.1052
Medical surveillance is provided for all employees who are or may be exposed to Methylene Chloride (MC), at or above the action level for 30 or more days per year. It is also provided for employees who are exposed at or above the Permissible Exposure Limit (PEL), or STEL for 10 days or more days during the year. Medical surveillance is also provided for any employee exposed above the PEL or STEL, who has been identified by a physician or other licensed health care professional as at risk for cardiac disease or some other serious MC-related health condition. Any employee may request inclusion, regardless of the duration of MC exposure. Medical surveillance is also available to all employees exposed to MC during an emergency.
(2) Frequency: The medical examination surveillance consists of a preplacement examination and annual examinations thereafter.
(3) Contents of the medical examination will include the following:
(a) A comprehensive medical and work history. This history includes an annual detailed work and medical history with special emphasis on cardiac history, skin conditions, history of hematologic disorder, liver disease, and MC exposures, work practices, and personal protective equipment used. Information should also be obtained from the worker regarding potential signs or symptoms associated with increased levels of carboxyhemoglobin. The examiner ensures that the smoking history of all employees exposed to MC is known.
(b) A complete physical examination with special emphasis on the lungs, cardiovascular system, liver, nervous system, skin, and vital signs. An evaluation of the employee's ability to wear a respirator.
(c) Laboratory surveillance: SMAC, CBC, U/A, ECG, PFT, visual testing.
(4) The OHP physician gives the affected employee a written opinion regarding results of the examination within 15 days of completing the evaluation of the medical and laboratory findings, but no more than 30 days after the initial examination. NASA receives notification that such written medical opinion has been provided to the employee. The written medical opinion is limited to the following:
(a) The physician's opinion as to whether the employee has any detected medical conditions that would increase the risk of material impairment from exposure to MC.
(b) Any recommended limitations on employee exposure to MC and on the use of personal protective clothing or equipment and respirators.
(c) A statement that the physician has informed the employee that MC is a potential occupational carcinogen, of the risk factors for heart disease, and the potential exacerbation of underlying heart disease from MC exposure and its metabolism to carbon monoxide.
(d) A statement that the physician has informed the employee of medical examination results and any medical conditions resulting from MC exposure requiring further explanation or treatment.
(5) The physician must not reveal to the employer, orally or in writing, any specific records, findings, or diagnoses that have no bearing on occupational exposures to MC.
(6) MRP benefits are required for employees removed from work exposure to MC, or for whom limited exposure to MC is recommended, due to existing/potential medical problems. MRP benefits are required if the physician finds that exposure to MC may contribute to or aggravate the employee's existing cardiac, hepatic, neur , ological or derma l symptoms.
, ,(7) Medical Records
(a) Will be kept for the duration of the employee's employment plus 30 years.
(b) In addition to demographics, the record contains the physician's written opinion and employee medical conditions related to MC exposure.
l. Methylene Diisocyanate, Methylene Triisocyanate
(1) Frequency: Complete preplacement physical examination and annual laboratory screening. Complete physical examinations a minimum of every 2 years.
(2) Laboratory: SMAC, CBC, U/A, PFT, visual testing.
m. 4, 4'-Methylene Bis (2-Chloroaniline) and 4,4'-Methylene Dianiline
(1) Reference: OSHA 29 CFR Part 1910.1050, 29 CFR Part 1926.62 (MDA) ASTDR Toxicological Profile on 4,4'-Methylene Bis (2-Chloroaniline)
(2) Frequency: Complete preplacement physical examination and annual laboratory. Complete physical examination a minimum of every 2 years.
(3) Laboratory: SMAC, CBC, U/A, visual testing, PFT, urine analysis for chemical content, and urinary cytological examination.
n. M-Phenylenediamine
(1) Frequency: Complete preplacement physical examination and annual laboratory screening. Complete physical examinations a minimum of every 2 years for workers exposed at or above the action level for 30 or more days per year.
(2) Laboratory: SMAC, CBC, U/A, PFT, visual testing.
o. Nickel
(1) Reference: NIOSH 77-164 1977
Inorganic nickel causes allergic eczema and probably is a cause of respiratory cancers. Soluble nickel compounds are irritants to the eyes, skin, and respiratory tract.
(2) Frequency: Complete preplacement physical examination and annual laboratory screening. Complete physical examinations a minimum of every 2 years for workers exposed at or above the action level for 30 or more days per year.
(3) Laboratory: SMAC, CBC, U/A, PFT
p. Polychlorinated Biphenyl
(1) Reference: NIOSH 77-225 1977
(2) Frequency: Complete preplacement examination and annual blood testing for serum PCB's as a minimum if there is a history of exposure, and ad hoc if significant exposure is documented or suspected.
(3) Special emphasis on hepatic and dermatological findings. Special work history for hazardous environments.
q. Thermal Ablative, Methylene Biphenyl Isocyanate
(1) Frequency: Complete preplacement examination and annual laboratory screening. Subjective and/or objective evidence of exposure requires complete examination. Complete examination at least every 2 years.
(2) Evaluation includes general occupational medicine history with special attention to the following:
(a) Previous exposure to isocyanates and other industrial chemical irritants.
(b) History of broncho/pulmonary disease or hypersensitivity.
(c) History of chronic eye or skin condition or hypersensitivity.
(d) History of anemia or other blood dyscrasia.
(e) Liver disease.
(f) Pulmonary function (forced vital capacity, forced expiratory volume in 1 second).
(g) CXR.
(h) Eye and vision examination with special attention given to cornea and conjunctiva.
(i) Chemistry battery with special attention to liver function tests.
(j) CBC.
(k) Urinalysis.
(l) Physical examination with special attention to evaluation of skin, lungs, liver, and spleen.
r. Trichloroethylene
(1) Trichlorethylene is classified as a probable human carcinogen.
(2) Employees having exposures above the action level for 30 or more days per year are included in the medical surveillance program.
s. Tungsten Carbide
Tungsten carbide tools used for cutting, drilling, sawing, and grinding contain cobalt. Dusts from this alloy can cause skin irritation and a variety of respiratory conditions. Employees assigned to work on a regular schedule with these devices are included in the medical surveillance program.
t. Noise (Hearing Conservation)
(1) Reference: OSHA 29 CFR Part 1910.95.
(2) The employee completes an appropriate baseline hearing questionnaire.
(3) Physical examination of the ear, nose, and throat system is accomplished prior to audiometry as deemed appropriate.
(4) An Audiogram is appropriately recorded.
(5) The examiner evaluates findings, past records, and recommends appropriate actions in accordance with dictates of good occupational health practice.
(6) Referrals for audiologic/otologic consultation are reviewed and approved by the OHP Medical Director or his designee prior to implementation.
(7) Hearing Evaluation Report Form Disposition and Hearing Conservation Registry:
(a) The original form is filed in the employee's medical record as a physical examination.
(b) The primary Hearing Conservation Registry consists of an alpha file of individuals.
(c) The annual hearing examinations are filed in individual employee's medical records.
(8) Each employee with a significant threshold shift is evaluated by a physician, preferably the physician overseeing the hearing conservation program.
(9) Each employee receives a letter detailing the results of the audiometric examination.
u. Silica Dusts
Crystalline or free silica inhalation may cause silicosis and is a mechanical irritant to the skin and eyes. It is categorized as a probable human carcinogen. Medical surveillance is recommended for employees exposed to crystalline silica at or above the action level for 30 or more days per year.
This section provides guidance for treating workers in potentially hazardous environments as detailed below.
a. Chemical Laboratory Workers
(1) Reference: OSHA 29 CFR Part 1910.1450.
This recommendation covers medical surveillance for workers having occupational exposure to hazardous chemicals in chemical laboratories. The laboratory is a workplace where protective laboratory practices and equipment are in place and relatively small quantities of hazardous chemicals are used on a nonproduction basis. This recommendation includes workers wearing "splash" protection that is not included in other physical examination category. It does not include workers in medical clinical laboratories.
(2) Frequency: Annually when employees are exposed to any specific regulated substance above the action level for 30 or more days per year, or if no action level is established, the PEL for 30 or more days per year.
b. Hazardous Waste Workers
(1) Reference: OSHA 29 CFR Parts 1910.120 and 1926.65.
(2) Frequency: Employees who are exposed to hazardous materials at levels greater than the action level for more than 25 days per year, who wear respiratory protection, or who have a significant potential for exposure through skin absorption, are provided initial, periodic, and terminal medical surveillance. Employees who may be covered by this program are employees involved in the transport, storage, and disposal of hazardous substances. This category also includes employees assigned to support hazardous operations other than those supported by the Hazardous Materials (HAZMAT) emergency response team. (The latter must also meet firefighter standards.) Some of these hazardous substances are as follows:
(a) Aromatic hydrocarbons.
(i) Benzene.
(ii) Ethyl benzene.
(iii) Toluene.
(iv) Xylene.
(b) Asbestos (or asbestiform particles).
(c) Halogenated aliphatic hydrocarbons.
(i) Carbon tetrachloride.
(ii) Chloroform
(iii) Ethyl bromide
(iv) Ethyl chloride
(v) Ethylene dibromide
(vi) Ethylene dichloride
(vii) Methyl chloride
(viii) Methyl chloroform
(ix) Methylene chloride
(x) Tetrachloroethane
(xi) Tertachloroethylene (perchloroethylene)
(xii) Trichloroethylene
(xiii) Vinyl chloride
(d) Heavy metals.
(i) Arsenic
(ii) Beryllium
(iii) Cadmium
(iv) Lead
(v) Mercury
(e) Herbicides.
(i) Chlorophenoxy compounds.
(ii) Dioxin
(f) Organochlorine insecticides.
(i) Chlorinated ethanes
(ii) Cyclodienes: aldrin, chlordane, dieldrin, endrin
(iii) Chlorocyclohexanes: lindane.
(g) Organophosphate and carbamate insecticides.
(i) Organophosphate: diazinon, dichlorovos, dimethoate, trichlorfon, malathion, methyl parathion, parathion.
(ii) Carbamate: aldicarb, baygon, zectran.
(h) Polychlorinated biphenyls.
(3) The medical surveillance program includes a complete physical and biological monitoring with pre-employment screening and periodic medical examinations as appropriate for the specific substances involved.
c. Insect and Pest Control
(1) Frequency: Complete preplacement examination, annual laboratory screening for pesticides and herbicides (bone marrow, liver, renal) and at least biennial physician examination. (See requirements in Section 3.2.3.2 concerning Hazardous Waste Workers.)
(2) Red blood cell cholinesterase is performed at least annually. (Recommended more frequently if handling organophosphates or carbamate pesticides on a regular basis.)
d. Spray Painting
Spray painters may encounter several hazardous chemicals. These include cadmium, chromium, lead, isocyanates, acrylates, organic solvents, epoxies, and glycol ethers. There may also be physical hazards such as heat, ergonomic stresses, working at heights, noise, and dusts.
e. Water and Sewage
(1) Frequency: Complete preplacement examination and annual laboratory screening.
(2) Immunizations may be provided on a voluntary basis but are not required.
(a) Typhoid - every 3 years.
(b) Tetanus/Diphtheria - every 10 years.
(c) Hepatitis A.
3.2.4 Job Certification Examinations
3.2.4.1 Introduction
Job certification examinations may be required by Federal or State statutes or by the employer. The Agency or organization establishing the requirement specifies the medical, laboratory and special procedure standards for these examinations. If the examinations are not performed onsite, the OHP Medical Director reviews the results before certifications are issued for doing the job.
3.2.4.2 Process Description
(1) The applicable medical standards for air traffic control are found in the FAA Guide for Aviation Medical Examiners (AME). (2) There are special concerns when providing health care to air traffic control employees. A physician with special training in aerospace medicine (either experience as a military or civilian flight surgeon or as an FAA AME, or training in military or civilian aerospace medicine residency programs) should always see these employees. (3) Two general concerns apply for air traffic controllers. The first is that a medical condition itself may prevent them from safely being able to fulfill their duties. The second concern is that the treatment for a condition, even though minor, may interfere with their ability to perform their normal duties. Air traffic controllers are not to control aircraft when administered medication that may affect their alertness or mental process. (4) Air traffic controllers are advised that they are not to perform these duties when being administered antihistamines, decongestants, tranquilizers, antidepressants, muscle relaxants, or sleeping medicines. (5) When a health care worker, other than a qualified aeromedical physician, sees an air traffic controller, the OHP Medical Director is notified and requested to recommend appropriate treatment and disposition. Documentation is maintained in the medical record and two copies given to the employees for their supervisor and for their own record. If it is appropriate that the employees can return to work, but not do air traffic controller duties, then their duty section or supervisor is notified. Employees are also told that they have to see OHP medical personnel for release to full duty when they are medically qualified. (6) All information related to complaints, physical examination findings, diagnosis, treatment, and disposition is recorded in the employee's medical record. If there is uncertainty with regard to disposition, the OHP Medical Director is consulted. b. Confined Space/Tank Entry (1) Frequency: Initial complete physical and age determined thereafter. Concomitant chemical or physical exposures may dictate that surveillance physicals also be performed. (2) Particular attention by the examining physician is directed to HEENT, neurological examination, and history to include
organic conditions predisposing to syncope, vertigo, convulsions. (3) Review of baseline laboratory data specific for detecting organic effects of the particular chemicals encountered. (4) Subject should be free of psychoemotional instability. c. Crane Operator/Ground/Floor/Remotely Operated Cranes (1) Frequency: Preplacement and age determined. (2) Additional visual requirements (See section 3.2.5): (a) Binocular vision. (b) Normal color discrimination (if colored signals are essential to operations). (c) Bifocal correction lenses are authorized. (3) Hearing: Hearing loss with or without aid, averaged in the frequencies of 500, 1000, and 2000 hertz (Hz) of no greater than 30 decibel (dB) in better ear. (4) If a question of cardiovascular fitness arises, evaluation by the employee's private medical doctor, and the appropriate documentation, may be needed. (5) Strength, endurance, agility, coordination, dexterity, and reaction speed consistent with normal, healthy physiology, and assigned task. (6) No history of seizures, emotional instability, or physical conditions or defects which could render the employee ineffective or a hazard to themselves, others, or the equipment being operated. d. Crane Operator/High (1) This section also includes Cab/Pulpit Operated Cranes. (2) Frequency: Preplacement and age determined. (3) Additional visual requirements (See section 3.2.5): (a) Binocular vision. (b) Normal color discrimination (if colored signals are essential to operations). (c) Bifocal correcting lenses are authorized, but larger near vision corrective segments are recommended. (4) Hearing: Hearing loss, with or without aid, averaged in the frequencies of 500, 1000, and 2000 Hz of no greater than 30 db in better ear. (5) Ability to complete 95 percent Predicted Age Adjusted Maximal Heart Rate (PAAMHR) of the Bruce Protocol* for treadmill exercise stress test without evidencing significant physiologic, electrocardiographic, or clinical pathology. (6) Strength, endurance, agility, coordination, dexterity, and reaction speed consistent with normal, healthy physiology for assigned task, ability to climb a vertical ladder. (7) No history of seizures, emotional instability or physical conditions or defects which could render the employee ineffective or a hazard to themselves, others, or the equipment being operated. e. Diver (1) Frequency: (a) Open water. Preplacement and age determined. (There may be frequency requirements that are unique to a requesting agency.) See Section f. (Down Range/Shipboard, and Remote Assignment). (b) Nonopen water. NASA Standard 8719.10, Standard for Underwater Facility and Non-Open Water Operations. (2) The following is included in the physical
examination: (a) A complete history is obtained from the employee. (b) Routine laboratory and CXR. (c) The examination by the physician includes a review of the history with elaboration of any positive findings, and further examination of the HEENT to rule out obvious anatomic problems, sinus difficulties, or dental disease. (d) Some employers may require long bone x-rays on initial examination. X-rays are performed on subsequent examinations only at the examining physician's request. (e) The employee completes 95 percent PAAMHR on the Bruce Protocol* without clinical and/or ECG signs/symptoms of significant cardiac disease. (f) A positive statement regarding ability to perform the Valsalva maneuver is noted. (g) The cardiovascular, respiratory, neuromuscular and orthopedic examination assure no gross abnormalities in those systems. (h) If there is reason to hold the medical qualification until additional medical evaluation is obtained, then inform the employee and NASA that the employee is not to dive until cleared by the OHP physician. (i) Examination for underwater facility and
non-Open Water operations require an examination by a certified (Hyperbaric or underwater) physician. *These are KSC requirements recommended Agencywide for Open Water divers. f. Down Range/Shipboard and Remote Assignments (1) The employee must be medically qualified for assigned job and have completed a pretravel questionnaire for travel clearance. (2) Frequency: Preassignment and age determined by the following directive: Downrange and Remote Assignment. The following schedule applies for Eastern Test Range Shipboard: *
Category/Age
Exam Cycle 17-24 years Every 5 years 25-49 years Every 3 years 50-59 years Every 2 years 60 years and up Annually Masters Annually Chief Mates Annually Chief Engineers Annually 1st Asst. Engineer Annually (3) Immunizations: (a) Immunizations may be required for all employees traveling outside the continental United States on official business. All
required immunizations are administered prior to departure. The OHP Medical Director confirms immunization requirements when appropriate. (b) Before administering immunizations to female employees, check their "pregnancy" status and determine what immunizations their physician has approved. The OHP physician may recommend a waiver of the immunization on the immunization record if clinically indicated. (c) Refer to the CDC "Blue Sheet" (weekly summary of countries with infected areas of Quarantined Disease and Health Information for International Travel) for current information on immunization requirements, access on Internet at http://www.cdc.gov. Immunization and country information can be obtained by contacting International SOS at 1-800-523-8930. (d) The following routine immunizations are recommended for travel to or on all range bases, sites, ships, outside the continental United States: (i) Measles, Mumps and Rubella (MRMR). (ii) Tetanus/Diphtheria every 10 years. (iii) Polio with one booster. (iv) Yellow Fever every 10 years. (v) Hepatitis B series. (Accompanied by the
appropriate bloodborne pathogen training. This vaccination series may be reserved only for health care workers, those assigned to first aid duties, or employees that are planning on extended tours of duty in areas where Hepatitis B is endemic.) (vi) Hepatitis A series. (4) Tuberculosis (TB) skin test status [Mantoux, Purified Protein Derivative (PPD) ] is documented prior to travel. If the PPD is positive (abnormal), then a physician examination and CXR are performed. If the employee has a documented history of a positive PPD,
then the physician determines if further testing is needed prior to clearance for international travel. If the CXR is abnormal and suggestive of active tuberculosis, then a consultation occurs to determine additional testing and treatment as appropriate. This information is documented in the record before travel clearance is approved. Post-travel followup for travelers with negative PPD is recommended to the employee. Refer to Section 3.4.5, Tuberculosis Prevention and Skin Testing, for more detailed information. (5) A records release form is inserted in each interim folder on SHIPSDUTY physical examinations. The OHP medical personnel obtain the employee's signature on the release form. (6) All female mariners are eligible for an annual Pap test, pelvic examination, and manual breast examination. Screening mammography according to ACS or American College of Obstetricians and Gynecologists recommendations is encouraged. (7) All remote assignment physical examinations include an initial blood type. Employees are informed of the blood type, and it is noted in the medical record. (8) All employees returning from a remote assignment with significant illness (hospitalization or early return due to illness) are disqualified for future remote assignment until approved by appropriate authority.* (9) Insulin-dependent diabetes mellitus is disqualifying for remote assignment.* (10) Noninsulin-dependent diabetes mellitus may be disqualifying for remote assignment, depending on the adequacy of treatment, side effects, end organ effects, and current health status of the employee.* (11) History of myocardial infarction, coronary artery bypass graft or percutaneous transthoracic coronary angioplasty is disqualifying for remote assignment.* NOTE: There are no waivers for the African transatlantic abort sites.* g. Federal Aviation Administration Personnel (1) Nonjob-related FAA physical examinations on NASA and contractor employees are performed at the discretion of the local contracting officer. FAA examinations that are job related or requested by the contracting office are performed for NASA and contractor employees. (2) Frequency is as required by FAA regulations. (See the FAA Guide for AME for applicable standards and examination procedures). (3) Only those test results required for the specific class of FAA requested physical are transposed to the FAA Form 8500-8. The following item notes apply: (a) Items 1 - 24: Medical Services personnel ensure applicant has completed FAA Form 8500-8. All blocks are completed. AME reviews for history, other relevant data, or omissions. (b) Items 25 - 48: Completed by AME. Review and apply the applicable chapter of AME Guide when abnormalities are reported. (c) Items 49 - 59: All elements completed by medical services personnel. h. Firefighter (1) Frequency: Preplacement and age determined. (2) All incumbent firefighters, firefighters/drivers, crew chiefs, and assistant crew chiefs receive complete physical examinations and medical certification. The following special requirements apply: (a) Audiological hearing aids are not permitted. (b) Psychologically, employee must possess mental and emotional stability. Any condition, which would cause applicants to be a hazard to themselves or others, is cause for disqu
alification. (c) Graded Exercise Test (GXT): Diagnostic symptom limited GXT (95 percent PAAMHR) at preplacement and on periodic physical examinations.* (d) Vision: Distant vision, uncorrected, tests at least 20/70 (Snellen) in one eye and 20/100 (Snellen) in the other eye, and corrected to at leas 20/20 in one eye and 20/40 in the other eye. Near vision corrected
, to 20/40 in both eyes.
i.
Food
Handler Use guidelines established for Primary Crew Contact described in section 3.2.5. j. Fuel Handler/Contingency Crew (1) Frequency: Complete preplacement with annual laboratory screening [blood (CBC), renal (U/A), liver (SMAC)]. (2) Employees must have the appropriate certifications to perform the basic job to which they are assigned. That is, their limitation or restriction permits them to function in the assigned position, e.g., employees with prosthetic arms would be excluded
from work on scaffolding. (3) Employees should be free of liver, renal, or significant cardiac or pulmonary disease that may be adversely affected by exposure to toxic propellants. Judgment of the OHP physician prevails, based, on considerations of examination, laboratory, and ancillary data. *KSC requirement recommended Agencywide. k. Hazardous Materials Emergency Response Team (1) Reference: OSHA 29 CFR Part 1910.120 and American National Standards Institute (ANSI) "Standard on Medical Requirements for Firefighters," National Fire Protection Association upgraded 1994. The response to HAZMAT usually comes from within the fire services and responds to actual or potential leaks or spills of hazardous substances. Members meet the requirements for Firefighter (See section 3.2.4.2.) and SCAPE (See section 3.2.4.2.) in addition to these special frequency examinations. (Hazardous waste workers, other than the HAZMAT emergency response team, meet the requirements for hazardous waste workers - see section 3.2.3.2) (2) Frequency: A complete physical at preplacement; every 2 years to age 50, then annually; at termination of HAZMAT exposure (if no physical examination performed within the previous six months),
and ad hoc for exposure occurrence. Annual surveillance examinations may be required if exposures dictate (See Section 3.2.3, Surveillance Examinations). l. Heavy Ordnance (1) This section also includes explosives handler. (2) Frequency: Preplacement and age determined (except military personnel and military contractors at Cape Canaveral Air Force Station, which are annually and at termination.)** (3) A history of seizures, emotional instability or physical conditions or defects which could render employees ineffective or a hazard to themselves, others, or the materials and equipment being handled or operated is considered disqualifying. m. High Crew/Spider (1) Frequency: Preplacement and age determined. (2) Special attention is directed to exclude any organic or psychological problems contraindicating work on high structures, or any organic problems contraindicating specific duty that the employee is required to perform on a high crew duty, e.g., painting, sandblasting, general mechanic duty. (3) Subject should be free of psychoemotional instability. n. Locomotive Engineer/Crawler-Transport (1) Frequency: Preplacement and age determined. (2) Employees should be free of electrocardiographic and physiologic signs/symptoms of significant heart disease and complete a 95 percent PAAMHR GXT, using the Standard Bruce Protocol.* * KSC requirement recommended Agencywide. ** KSC unique. o. Motive (Heavy) Equipment Operators (1) Any vehicle over one ton is considered to be heavy equipment. (2) Frequency: Preplacement and age determined. (3) There should be no significant medical problems that might lead to sudden incapacitation, such as seizure history, cardiovascular disease or, diabetes mellitus. (4) There should be no history of emotional instability or physical conditions or defects, which could render employees ineffective or a hazard to themselves, others, or the equipment being used. p. Multiple Passenger Vehicle Operator (1) Reference: 49 CFR Part 391.41. (2) Frequency: Preplacement and age determined to comply with DOT Commercial Drivers License requirements. (3) Examination includes a GXT. The employee must achieve as a minimum 95 percent of their PAAMHR without exhibiting any electrocardiographic or physiologic evidence of significant cardiovascular disease.* *KSC requirement recommended Agencywide. q. Vehicle Certification (1) Reference: 49 CFR Part 391.41. (2) Frequency: Every 2 years (biannually). (3) DOT physical examination standards apply. Employees may be physically qualified if they-- (a) Have no loss of a foot, a leg, a hand, or an arm, or have been granted a waiver. (b) Have no impairment of 1) a hand or finger which interferes with prehension or grasping; or 2) an arm, foot, or leg which interferes with
their ability to perform normal tasks associated with operating a motor
vehicle; or 3) any other significant limb defect or limitation which interferes with their ability to perform normal tasks associated with operating a motor vehicle; or 4) have been granted a waiver. (c) Have no established medical history or clinical diagnosis of diabetes mellitus currently requiring insulin for control. (d) Have no current clinical diagnosis of myocardial
infarction, angina pectoris, coronary insufficiency, thrombosis, or any
other cardiovascular disease of a variety known to be accompanied by syncope, dyspnea, collapse, or congestive heart failure. (e) Have no established medical history or clinical
diagnosis of a respiratory dysfunction likely to interfere with their ability to control and drive a motor vehicle safely. (f) Have no current clinical diagnosis of high blood
pressure likely to interfere with their ability to operate a motor vehicle
safely. (g) Have no established medical history or clinical
diagnosis of rheumatic, arthritic, orthopedic, muscular, neuromuscular, or
vascular disease, which interferes with their ability to control and operate a motor vehicle safely. (h) Have no established medical history or clinical
diagnosis of epilepsy or any other condition, which is likely to cause loss of consciousness or any loss of ability to control a motor vehicle safely. (i) Have no mental, nervous, organic, or functional disease or psychiatric disorder likely to interfere with their ability to drive a motor vehicle safely. (j) Have distant visual acuity of at least 20/40 in each eye without corrective lenses or visual acuity separately corrected to 20/40 or better with corrective lenses. Field of vision of at least 70 degrees in the horizontal meridian in each eye, and the ability to recognize the colors of traffic signals and devices showing standard red, green, and amber. Monocular employees are not qualified to operate commercial motor vehicles. (k) Can perceive a forced whispered voice in the better ear at not less than five feet with or without the use of a hearing aid or if tested by use of an audiometric device. (l) Do not have an average hearing loss in the better ear greater than 40 dB at 500 Hz, 1,000 Hz, and 2,000 Hz with or without a hearing aid when the audiometric device is calibrated to ANSI standards. (m) Do not use Schedule I drugs, an amphetamine, narcotic, or any other habit-forming drug. The exception would be that employees might use such a substance or drug if an OHP physician who is familiar with their medical history and assigned duties prescribes it. In addition, the OHP physician advised the employees that the prescribed substance or drug would not adversely affect their ability to safely
operate a motor vehicle. (n) Have no current clinical diagnosis of alcoholism. (4) Employees who are deemed not medically qualified to operate motor vehicles according to the above criteria are considered for a waiver only in the following circumstances: (a) Loss of a foot, leg, hand, or arm with little residual impairment of the employee's ability to safely operate motor vehicles. (b) Impairment of a hand or finger that interferes with grasping, if the impairment does not impair the employee's ability to safely operate motor vehicles. (c) Impairment of an arm, foot, or leg that does not
interfere with the employee's ability to safely operate motor vehicles. (d) Any other significant limb defect or limitation, which does not significantly interfere with the employee's ability to safely operate motor vehicles. (5) Requests for DOT waivers are submitted by the employee and the employer to the Regional Director, Federal Motor Carrier Safety. r. Noncrew Flying (1) Preassignment and age determined. (2) This procedure is followed for physical examinations on NASA and contractor employees who are required to fly on NASA aircraft as noncrew members in performance of their jobs. These include fire, security, safety, photo-optics, and other observer
personnel. (3) The scope of the physical examination is the same as that required for FAA Class III. The results are recorded in the medical record or on an approved form. The FAA Forms are not to be used. (4) The records of employees who do not meet the standards for FAA Class III examination in accordance with the FAA manual are referred to the OHP Medical Director for review. An entry in the medical record is made by the examining physician to state the reason(s) for disqualification. This note in the medical record will also cite the reference for disqualification from the FAA Guide for AME. (5) The OHP Medical Director may determine that the reason for the disqualification would not threaten the well being of the employee or the mission and grant a waiver to perform the assigned duties. s. Selfcontained Atmospheric Protective Ensemble (SCAPE) (1) This section also includes the Self Contained Breathing Apparatus and the Liquid Air Pack. (2) Reference: OSHA 29 CFR Part 1910.134 (The OSHA Respiration Protection Standard is applicable to two physical examination categories): (a) SCAPE, which addresses the requirements for employees assigned to enter atmospheres that are immediately threatening to life and health or are expected to perform rescue/evacuation operations. (b) Other employees that are assigned to jobs that necessitate the wearing of respiratory protection other than SCAPE [See section 3.2.5]. (3) Frequency: Preplacement and age determined (for employees assigned to enter atmospheres that are immediately threatening to life and health or are expected to perform rescue/evacuation operations). (4) General considerations: (a) It is recognized that the SCAPE suit, although a protective garment, can, under certain circumstances, present a significant hazard to employees who are not in good physical condition or who have some medical or physical limitations. (b) An inherent requirement is for employees to be physically able to evacuate an area quickly while in the SCAPE suit. Evacuation might entail rapid ascent or descent of stairs or a ladder or even to run some distance and might require that the employee assist another adult in rapid egress. (c) The first or basic requirement for SCAPE suits is that employees have no gross physical defect that would prevent normal mobility, performance of job, ability to evacuate an area rapidly, or to assist another to the point of supporting a major part of another adult's weight. (d) The second general concept is that employees are able to see well enough with correction to perform their usual tasks and to be able to see well enough without correction to evacuate a position under adverse circumstances such as relative darkness. (e) The third basic principle is that employees have no chronic or active disease process that has a reasonable probability of resulting in an emergency evacuation of employees or their partners in the buddy system, or of adversely impacting the integrity of the operation. (f) A fourth principle applies to employees who are theoretically in the high risk group for acute cardiopulmonary and vascular emergencies who might not have an otherwise disqualifying condition. For this last consideration, the determinant is the judgment of the examining physician, after performing complete physical examinations with special cardiovascular testing for SCAPE suit duties. (5) Specific requirements for SCAPE duty qualifications: (a) GXT is performed at each examination (Completes stage III of Bruce Protocol without significant electrocardiographic or physiological abnormalities).* (b) Acceptable visual acuity, as outlined in the section 3.2.5.) (c) Normal depth perception is not required unless it is a specific requirement of the employee's duty while in SCAPE suit, e.g., crane operator. t. Security Personnel (1) Frequency: Complete physical preplacement and age determined. (2) Visual standards apply as listed in section 3.2.5. (3) GXT: Completes 95 percent PAAMHR on the Bruce Protocol without clinical and/or ECG signs/symptoms of significant cardiac disease. Consideration is given to those employees who are on beta blockers in regards to maximum heart rates achieved on the GXT.* (4) The OHP physician must recognize that other security duties, such as Special Weapons And Tactics (SWAT) team duties, require more stringent physical activities and may not be appropriate for certain employees. Such factors must be considered when certifying
security personnel. *KSC requirement recommended Agencywide. u. Solid Rocket Booster Retrieval (1) Frequency: Complete physical, preplacement and age determined (2) Visual standards apply as listed in. section 3.2.5. (3) Certifications for other duties (section 3.2.4.2 Diver, Down Range) are current. 3.2.5 Special Purpose Examinations 3.2.5.1. Introduction Centers of other agencies/contractors authorized to use services provided by the OHP facilities may request examinations that are not included in any of the other categories. Examples of these types of examinations are disability, retirement, termination, Fitness For Duty (FFD), Return To Work (RTW), independent medical examination, and primary crew contact. 3.2.5.2 Process Description 3.2.5.3 Employment Situations a. Fitness for Duty - General FFD examinations are usually performed when employees are returning to work after an illness or injury. These examinations may also be requested when a change in the employee's health or performance is observed or suspected. Determination of fitness requires identification and documentation of the physical requirements and essential functions for the job. Firsthand familiarity with the job by the OHP physician is invaluable. b. Fitness For Duty - Civil Service (1) The office requesting the FFD examination indicates on Standard Form (SF) 88 the functional and environmental factors required for the employee to meet the standards for the job classification. (2) The office requesting the FFD examination obtains the employee's consent to have an FFD physical prior to the examination. (3) If possible, the examination is completed in 1 day. (4) A cover letter from the requesting office accompanies the SF 88 with instructions for completion and includes the forwarding procedure for the completed SF 88. c. Fitness For Duty - Contractor FFD examinations are accepted when the requesting employer or employee meets the following criteria: (1) A job description that specifies physical performance requirements accompanies the request. (2) The proposed employee has been previously examined under and has met the requirements of the published Physical Examination Standard applicable to the job for which fitness is questioned. (3) A supervisory statement accompanies the request that describes how the employee does not measure up to the requirements of the job. (4) The employer requires the employee to obtain clearance for RTW from OHP medical personnel after an illness/injury and that this clearance is recorded in the employee's medical records retained by OHP medical personnel. (5) Requests for FFD examinations not meeting all stated criteria are returned to the originator without action. d. Return To Work (1) RTW physicals are requested by authorized users of the OHP medical services. The procedures are as follows: (a) The OHP Medical Director receives a "Letter of Instruction" and two copies of SF 88 from the office requesting the physical. (b) The physical is scheduled, and the requesting office is notified of the date and time. (c) The employee has an interview with an OHP physician, who ascertains the required extent of the evaluation that is needed. (d) After the employee's examination, the OHP physician fills out the duplicate copy of the SF 88 and forwards it to the requesting office. (2) Most other RTW physicals do not require a full physical examination. OHP medical personnel determine the extent of the examination and appropriate laboratory testing. If fasting laboratory work is needed, the employee is scheduled for a followup examination as required. RTW certification and statement of limitations (and duration), if any, are documented along with recommendations for disposition. These are distributed as follows: (a) Original given to employee to give to his supervisor. (b) Copy to Company Safety Officer, for occupationally related issues.
(c) Copy filed in employee's medical record. 3.2.5.4 Visual Examinations a. General Examinations (1) Two types of functional eye examinations are given for job certifications during partial or complete physical examinations: visual acuity and color perception. Administration of these tests is suggested for such positions as Quality Control Inspectors, Electricians, Welders, Solderers, Crane Operators, or any other personnel who may be required to have specified visual acuity and distinguish color vision as part of performing their normal job duties. Depth perception and visual fields may also be required. (2) Job classifications that require Federal Aviation Administration (FAA) and Department of Transportation (DOT) certifications have differing and additional requirements. It is recommended that FAA and DOT regulations be consulted for actual requirements. b. Specific Recommendations (1) For all complete physicals record at least the visual acuity and color perception. (2) Visual acuity should be evaluated with tests such as Snellen (far) and Jaeger (near). (3) Color perception should be evaluated with tests such as Dvorine Plates or Ishihara Plates. (4) Frequency: Annual examinations are suggested, but individual Centers may establish more frequent requirements. 3.2.5.5. Work Place Exposures a. Bloodborne Pathogens (1) Reference: 29 CFR Part 1910.1030 (more detailed information follows in Section 3.4, Infection Control). (2) Frequency: For BBP, initial Hepatitis-B vaccine is offered to all employees identified by a company's Exposure Control Plan (as required by Federal Register 29 CFR Part 1910.1030). Annual training is also required by the applicable CFR. (3) If employees decline the vaccine, they sign a mandatory declination statement. If at a future time an employee chooses vaccination, then a resubmission request for vaccination is honored. (4) A copy of the declination statement for those who decline vaccination for Hepatitis B is maintained. Access to the record for inclusion in the Exposure Control Plan documents is permissible. (5) All employees who are occupationally exposed to BBP's are evaluated according to the current CDC guidelines. b. Laser Workers (1) References: AFOSH Std. l6l-l0 dated 5/30/80, "Health Hazards Control for Laser Radiation", ANSI Z136.1 - 1986 "Safe Use of Laser." (2) Frequency: As determined by risk (see below). (3) The employer places all employees whose duties require their routine presence within the minimal safe distance of a laser/laser system (as defined by the Radiation Protection Officer) in a medical surveillance program. The extent of the surveillance program is determined in part by the following: (a) The risk classification of the employees as assigned by the Radiation Protection Officer. (b) The wavelengths of radiation being utilized. These parameters are specified to the medical facility by the requesting organization so that the appropriate minimum test requirements may be selected. This does not include visitors who are adequately protected by appropriate protective eyewear or other administrative/procedural controls. (4) Laser worker risk classification categories are as follows: (a) Incidental Personnel - Exposure possible but unlikely that they will be exposed to laser energy sufficient (Class IIIb and IV) to cause physical damage, e.g., custodial, clerical, supervisory personnel. (b) Laser Workers - Personnel who work routinely in laser environments with higher powered lasers (i.e.,Class IIIb and up) and are ordinarily protected by engineering/procedural type controls. (5) Medical surveillance for incidental personnel includes baseline visual acuity. Laser workers receive ocular and medical histories, visual acuity, Amsler Grid Test, and color vision tests as described below. (a) Ocular History. The past eye history and family history is reviewed. Any current complaints related to the eyes are noted. Inquiry is made into the general health tatus with a special emphasis upon systemic diseases that may produce ocular problems. Use of corrective lenses is recorded. Certain medical conditions may cause the laser worker to be at an increased risk for chronic exposure. Use of photosensitizing
medications, such as phenothiazines and psoralens, may lower the threshold for biological effects in the skin, cornea, lens and retina. Aphakic individuals would be subject to additional retinal exposure from blue light and near ultraviolet and ultraviolet laser radiation. Unless chronic viewing of these wavelengths is required, there should be no reason to deny laser operations to these aphakic individuals. (b) Visual Acuity. Visual acuity for far and near vision is measured according to established procedure. Refraction corrections should be made if required for both distant and near test targets. If visual acuity does not correct to 20/20 for distance and Jaeger 1 for near, an examination is required as listed in section 3.2.5. (c) Macular Function. An Amsler grid or similar pattern is used to test macular function for distortions and scotomas. If any distortions or missing portions of the grid pattern are present, the test is not normal.
(d) Color Vision. Color vision discrimination is documented by Ishihara or similar color vision tests. (e) Examination of the Ocular Fundus with an Ophthalmoscope. This portion of the examination is administered to
individuals whose ocular function tests, which are described above, are
not normal. The points to be covered include the presence or absence of
opacities in the media; the sharpness of outline of the optic disc; the color of the optic disc; the depth of the physiological cup, if present; the ratio of the size of the retinal veins to that of the retinal arteries; the presence or absence of a well-defined macula and the presence or absence of a foveal reflex; and any retinal pathology that can be seen with an ophthalmoscope (hyperpigmentation, depigmentation, retinal degeneration, exudate, as well as any induced pathology associated with changes in macular function). Small deviations from normal should be described and carefully localized. Dilation of the pupil is required. (f) Skin Examination. Examination of the skin is not required for preplacement examinations of laser workers; however, it is suggested for employees with history of photosensitivity or working with ultraviolet lasers. Any previous dermatological abnormalities and family history is reviewed. Any current complaints concerned with the skin are
noted as well as the history of medication usage, particularly concentrating on those drugs which are potentially photosensitizing. (g) Other Examinations. Further examinations are performed as deemed necessary by the OHP physician. (6) Medical Referral Following Suspected or Known Laser Injury: Any employee with a suspected laser eye and/or skin injury should contact the Occupational Health facility. The postexposure examination includes items as listed above as deemed necessary by the OHP physician. (7) Records and Record Retention: Complete and accurate records of all laser medical examinations are maintained for all personnel included in the medical surveillance program. Records are retained for at least 30 years. The
results of medical surveillance examinations are discussed with the employee. c. Primary Animal Contact (1) All requests for primary animal contact are
, approved by the NASA Center Contracting Officer's Technical Representative's Office. (2) Classification: (a) Access (b) Direct (c) Primate (3) Frequency: Annually (4) A comple
, te history is recorded. (5) Laboratory
, procedures: All require SMAC, CBC, U/A, C-reactive protein, Rapid Plasma Ragin (RPR) and stool for bacterial examination for Salmonella and Shigella. (Frequency of stool specimen: Initial and at the discretion of the OHP physician). (6) Immunizations: Tetanus/Diptheria every 10 years or sooner if clinically indicated and other vaccines as indicated. (7) PPD: Not more frequently than annually. The PPD is not performed on employees with a known/documented, previously positive test. If PPD is abnormal, a followup CXR is performed to survey for possible TB disease. Followup CXR in subsequent years is at the discretion of the OHP physician. (See section 3.2.4.2, Immunizations). (8) Examination by an OHP physician to include checking the employee's hands, face, neck, scalp, nose, throat, and feet to determine absence or presence of infectious disease. d. Primary Crew Contact (1) Standards are established in the Health Stabilization Program administered at Johnson Space Center (JSC). (2) Frequency: Crew food handlers and crew quarter
custodians every six months. All others annually. Expiration of the
certification is the last day of the month in which the physical examination was completed, plus six or 12 months, whichever applies. (3) Use JSC Forms 115A, 115B, 115C (4) Laboratory procedures: (a) RPR, Serum Glutamic-Oxaloacetic Transaminase (SGOT), CBC with differential, U/A. (b) Additional laboratory requirements for Food Handlers: (c) Stool culture for Salmonella, Shigella, and examination of the stool for ova and parasites. (d)Throat culture for significant respiratory pathogens such as Beta-hemolytic and Streptococci. (5) Immunization requirements: (a) Tetanus/Diptheria every 10 years. (b) MMR if no history of immunization or negative serologies. (c) Influenza and Hepatitis A vaccination is offered but not required. (d) PPD: Annual PPD screening required. The PPD is used except for those with a history of a positive PPD. The OHP physician reviews all positive PPD tests. CXR is indicated for all positive PPD's. With a history of a positive PPD, annual CXR examinations are at the discretion of the OHP physician. (6) All employees with this certification view the Primary Crew Video. (7) Temporary Disqualification: Whenever an employee
on the Health Stabilization Program is temporarily disqualified/decertified due to illness, their Primary Crew Contact badge is held by OHP medical personnel. When the employee is cleared to return to full duty, the badge is returned to the employee. e. Ionizing Radiation Workers (1) Frequency: Stated below, depending upon examination category, the radiation medical examination includes, but is not limited to, a careful history, complete physical examination, CXR, CBC, U/A, SMAC and other bioassays as indicated by the OHP physician. If a physical examination has been conducted within the previous six months and has been duly recorded in the employee's health record, it may, at the discretion of the OHP physician, be accepted in whole or in part in lieu of the corresponding sections of the radiation medical examination. Complete examinations conducted more than 6 months previously, may be utilized with appropriate supporting information and a signed interval note by the cognizant OHP physician in line 73 of the SF 88 (or equivalent). A physical examination conducted for one purpose is valid for any other purpose within the prescribed validity period if that physical contains the proper data. If the examination is deficient in scope, only those tests and procedures needed to meet the additional requirements are performed.
The results are recorded, and the OHP physician signs the appropriate approval. (2) Types of radiation medical examination are as follows: (a) Preplacement examination: All employees who are being considered for assignment to duties or occupations requiring exposure to ionizing radiation or the handling of radioactive material are given a medical examination prior to assignment or transfer to those duties or occupations. This examination is performed to ensure that a rospective employee is physically qualified for occupational exposure to ionizing radiation. People who are not routinely exposed to ionizing radiation during their normal occupations and who are not likely to exceed 0.5 roentgen-equivalent-man (rem) per year or 0.125 rem per quarter are not
required to have preplacement examinations. These people include visitors,
messengers, servicemen, delivery men, and certain crewmembers or employees
whose exposure is truly sporadic. (b) Re-examination: Employees who are exposed to ionizing radiation in the course of usual duty or employment are examined every 3 years. These examinations are required to ensure that employees receiving occupational exposures above the limits permitted by the general population continue to meet the physical standards. Employees who receive less than 0.5 rem each year are exempt from reexamination. (c) Situational examination: A special medical examination is given as soon as it can be scheduled for any employee who has exceeded the current radiation protection standards for occupational exposure, or has possibly ingested or inhaled a significant amount of radioactive material, or as deemed necessary by the OHP physician. (d) Termination examination: All employees who have
received greater than 0.5 rem in any 1 calendar year are given a radiation
physical examination at the termination of their employment. These
examinations are required in order to verify the physical status of employees at the end of their employment. Upon transfer from duties as radiation workers, an entry is made on SF 88 (or equivalent) that reflects the following statement: "Termination Radiation Physical Examination required prior to release or retirement". (3) Medical history: For the preplacement examination, a complete medical history is obtained. The medical histories on all employees receiving radiation medical examinations specifically include the following: (a) History is completed on each employee. (b) Work history to determine the amount of ionizing
radiation previously received as a result of occupational exposure. (c) Past history to evaluate any previous malignancies or pre-malignant lesions. (d) History of radiation therapy that may have been
previously received. (e) Family history of malignancies, pre-malignant lesions, infertility, cataracts, and congenital or familial defects. (f) For reexaminations, the medical history may be limited to that interval of time since the last radiation physical examination. The OHP physician is alert for symptoms of chronic illness and anxiety regarding exposure to radiation. (4) For situational examinations, the medical history may be limited to a detailed account of the employee's activities at the time the exposure occurred and to an interval history since the last radiation physical examination. Evidence of malignant and pre-malignant lesions, lenticular opacities, and other conditions that could be related to radiation is elicited. Positive entry in the documentation record describes ophthalmoscopic examination, e.g., "media clear" or "no lenticular opacities", or if no clear findings are apparent. (5) If lenticular opacities are identified, slit lamp
examinations are performed. All employees who are 36 years of age or older
shall have a slit lamp examination. Slit lamp examinations are conducted by an ophthalmologist and properly documented. (6) Lenticular opacities in the following categories are submitted to the OHP Medical Director for review: senile, traumatic, or metabolic, in a posterior subcapsular location. Opacities in the following categories need not be submitted for review (unless in a posterior subcapsular location): punctate pigment, vacuole, Y suture, hyaloidea
artery remnant, and Mittendorf spot. (7) Laboratory Procedure are as follows: (a) CXR required on all preplacement examinations (b) CBC with differential, U/A, and SMAC. f. Respirator, Occupational, (Non-SCAPE) (1) Reference: 29 CFR 1910.134 OSHA Respiratory Protection Program Standard [See section 3.2.4, s., Self Contained Atmospheric Protective Ensemble (SCAPE), for more information.] (2) Frequency: Initial medical evaluation and subsequent examinations depend upon the type of respirator and conditions under which a respirator is used, as well as the medical conditions/problems that are identified during the medical evaluation. All respiratory users in this category have at a minimum a medical questionnaire update and medical evaluation every 5 years. Employees with identified medical problems that can be assumed not to be static conditions, may be reevaluated on a more frequent schedule. The certifying examiner or OHP physician determines the frequency. (3) General considerations are as follows: (a) Respiratory protection equipment is worn to protect employees from a hazardous environment. The character of the hazardous environment and the above medical evaluation frequencies determine the frequency and extent of the medical evaluation. The specifics of the type of respirator used, the environment under which those respirators are used, and the frequency of use is all covered in the Respirator Medical Evaluation Questionnaire. (b) In addition to the hazardous environment in which
employees may work, respiratory protection equipment itself may produce an
abnormal physiologic demand upon the body, and add to the psychological and
physical stresses that exist. Devices such as SCAPE suits add considerable
weight and increased physiologic demands on the cardiovascular, pulmonary, and musculoskeletal systems. Positive pressure demand systems complicate the normal physiology of breathing and add additional pulmonary, cardiovascular, and physiological stresses. The respiratory protective gear or exposure to environmental hazards may aggravate certain existing abnormalities. All these factors are considered in the physical and psychological screening examinations. (c) The screening physical evaluation is accomplished prior to training and assignment to a respiratory protection position. It identifies medical and psychological problems that could jeopardize the welfare of the employee, coworkers, or the operation to which the employee is assigned. (4) Special requirements for Respiratory Medical Certification: A medical and work history to identify medical conditions that may adversely affect the employee's ability to safely use a respirator is covered with the use of the Respirator Medical Evaluation Questionnaire. An additional medical evaluation is required for any positive answers to questions on the questionnaire. This medical evaluation is of such degree that it provides enough additional information to aid the OHP physician in making a determination of whether the employee can safely wear and operate with the requested respirators. (5) The Respirator, Occupational, Non-SCAPE certification includes wearing the following respirators: Half Face Air Purifying, Full Mask Air Purifying, Half Face Partial Air Purifying Respirator (PAPR), Supplied Air Respirator (SAR), Full Face PAPR, SAR Helmet/Hood, SAR Half Face Continuous Flow, SAR Full Face Continuous Flow and SAR Positive Pressure. (6) Employees working in these devices need to demonstrate general good health. Any positive responses on the questionnaire that indicate problems with cough, phlegm, asthma, episodic wheezing, diabetes, seizures, or claustrophobia need to have OHP physician evaluation. Cardiovascular problems need to be well defined and determined to be under adequate control, without acute problems such as congestive heart failure or angina. (7) No allowed waivers for respirator usage are expected. OHP physicians' concerns/questions about specific respirator use can result in field testing of the respirator, permitting additional information for the OHP physician to make a final determination of medical certification. Field testing normally is used only on rare occasions. g. Respirator, Other (1) Reference: 29 CFR 1910.134, OSHA Respiratory
Protection Program Standard. (2) [See section 3.2.4, s, Self-Contained Atmospheric Protective Ensemble (SCAPE) for more information.] This category of examination applies to employees who are not assigned regularly to work in any environment presenting a respiratory hazard. They may be advised to wear a dust mask or filtering face piece as a personal protection on a temporary basis. They may need training and appropriate certification for these tasks, and hence medical clearance. (3) NOTE: OSHA has excluded Emergency Life Support
Apparatus respirators from the medical requirements in the 29 CFR 1910.134 standard. (4) Frequency: Initial medical evaluation and subsequent examinations at a frequency, depending upon the medical conditions/problems that are identified during the initial evaluation. All respiratory users in this category have at a minimum a medical questionnaire update and medical evaluation every 10 years. Employees with identified medical problems, which can be assumed not to be static conditions, may be reevaluated on a more frequent schedule. The certifying examiner or OHP physician determines the frequency. (5) General considerations are as follows: (a) Respiratory protection is worn to protect the worker from a hazardous environment. The types of respirators and conditions for use are identified in the Respirator Medical Evaluation Questionnaire. (b) The screening physical evaluation is accomplished prior to training and assignment to a respiratory protection position. The evaluation identifies medical and psychological problems that could jeopardize the welfare of the employee, coworkers, or the operation to which the employee is assigned. (6) Special requirements for respiratory medical
certification: A medical and work history to identify medical conditions that may adversely affect the employee's ability to safely use a respirator are covered with the use of the Respirator Medical Evaluation Questionnaire. An additional medical evaluation is required for any
positive answers to the questions on the questionnaire. The medical evaluation is of such degree that it provides enough additional information to aid the OHP physician making a determination of whether the employee can safely wear and perform required functions using the requested respirator. (7) Respirator users in this category need to be in
generally fair condition with no,significant limiting physical factors that would duly limit their or other employees ability to perform a proper egress. h. Tuberculosis Control (1) Frequency: Annually in health care workers. Biannually in daycare workers. (2) Program is limited to administration, documentation and interpretation of TB skin testing and appropriate followup for positive tests (or a history of a positive skin test). (3) Employees with a history of a positive PPD generally have an annual CXR; however, this practice is at the discretion of the OHP physician and is often omitted if there are no signs or symptoms of the TB disease in the employee. The findings and recommendations must be fully documented. 3.2.6 Health Maintenance Examinations 3.2.6.1 Introduction Health Maintenance examinations are voluntary and very effective in maintaining a healthy workforce if incorporated into NASA's overall wellness/health education program. The purpose of NASA's Health Maintenance examination is to maintain a healthy workforce by identifying an individual's potential health risks in order to educate, modify behavior, or refer out for further treatment. The purpose of standardizing these exams is to ensure that all employees have access to the minimum acceptable services that will be the same from Center to Center. An employee transferring from one Center to another will be assured of finding the same basic services and compatible baseline information. 3.2.6.2 Process Description< a. Federal Employees Health Program (FEHP) (1) FEHP examinations on civil service personnel are conducted according to the criteria established in this document. (2) Frequency: Complete physical examinations are given every 3 years, with appropriate initial baseline and interim history. A limited examination may be offered in the interim years. (3) Complete physical examinations includes the following: (a) Examination by, or under the auspices of, a physician (including an offer of a total body skin examination, prostate and testicular, or pelvic and breast examinations). (b) Vital signs - including height, weight, blood pressure, pulse rate and rhythm. (c) Fasting blood chemistry profile to include glucose and complete lipid profile (total cholesterol, HDL, LDL, triglycerides and total cholesterol/HDL ratio with CBC). (d) Urinalysis. (e) Hemocult. (f) Visual acuity.
(g) 12-lead ECG. (h) Pap test for females and PSA for males. (i) Mammograms with periodic followup as described below. (j) Baseline examinations with followup examinations if clinically indicated (audiogram, chest x-ray, pulmonary functions with Forced Vital Capacity (FVC), Forced Expiratory Volume (FEV) 1, and Forced Expiratory Flow (FEF) 25-75. (k) Baseline cardiac stress test and/or sigmoidoscopic or colonoscopy (if indicated by history and examination
findings) examination should be offered (within the clinic if NASA standards are met, or from outside resources if unavailable within the clinic), and followup as clinically indicated. (l) Ocular tonometry for glaucoma (in the clinic if using an air pressure instrument or an outside resource otherwise). (m) Optional tests offered if clinically indicated may include a thyroid profile including Thyroid Stimulating Hormone (TSH) (with T3 and T4 if TSH abnormal or clinically indicated), skin-fold or BMI. (4) Limited or partial physicals are offered in the interim years and include an examination and interim history, vital signs, the complete blood chemistry, CBC, urinalysis, hemocult test, or other elements of a screening battery. Females are offered pelvic/pap tests and breast examinations, and males are offered prostate and testicular
examinations annually. Mammograms are offered according to the following
guidelines: (a) 35 and older - initial baseline. (b) 40-49 - every 2 years. (c) 50 and older - annually. (5) Those who have had breast pathology or breast surgery, including breast implants, should have mammograms at a fixed medical facility rather than with a screening examination in a mobile unit. (6) Additional tests and/or examinations may be requested at the discretion of the examining official. (7) Employees, upon written request, may have the results of their examination furnished to their private physician. (8) Eligibility of other Federal employees (DOD, National Park Service, and Fish and Wildlife Service employees) may be eligible for both the Health Maintenance examination and job-related
occupational physical examinations (eligibility is determined by individual
contracting organizations). b. NASA Executives (1) Personnel designated as NASA Executives for physical examinations request their physical examinations in the same manner as for the job-related physical examinations. (2) Complete and limited physicals for NASA Executives are similar to those described in paragraph a. 3.3.1 Life Support Services 3.3.1.1 Introduction a. Life support services are generally provided as emergency measures. These services are configured to respond to onsite injuries and illnesses, to deal with immediate life threatening conditions, and to stabilize the patient for expeditious transportation to more comprehensive medical services when indicated. b. Every conceivable situation must be envisioned and a deliberate response designed. Many situations may be definitively managed with resources provided onsite. When circumstances beyond the capabilities of onsite occupational health services are encountered, plans and expedient action must ensue that the patient and additionally required services be brought together. 3.3.1.2. Responsibilities a. Each NASA Center must plan for and implement at the widest level possible provision to furnish, as the minimal response, Basic Life Support in the timeliest manner feasible. All clinic personnel, including receptionists, and secretaries must have BLS/CPR training. Health professionals must be trained and have medical equipment to provide Advanced (cardiac and/or trauma) Life Support (ACLS) on scene (preplacement when indicated by nature of hazard, as Shuttle launch and landing support) or by rapid response teams (fire and rescue, ambulance teams). b. Readiness of such capabilities will be assured by
appropriate and current certifications of personnel, by date verification of equipment and medications, and by periodic training exercise of the emplaced capability. To assist maintenance and readiness of equipment and medications, an Emergency Crash Cart Checklist is found in Appendix E. c. Reserved. 3.3.1.3 Process Description a. Adequate provision of emergency response must first determine the extent and nature of probable requirements. This is followed with deliberate decisions as to what level and types of response will be furnished onsite, which conditions will be transported to other medical capabilities, and what resources will be required to deliver those responses. b. Plans, personnel, and equipment must be provided. Plans shall be periodically reviewed for acceptability and currency, personnel will be appropriately trained and certified, equipment and supplies will be maintained and verified functional and current, and training simulations of total response systems will be periodically exercised. Services and their readiness must be documented. 3.3.1.4 References NPR 8715.2, NASA Emergency Preparedness Plan Procedures and Guidelines. 3.3.1.5 Flow Diagram The flow diagram for this process is shown in Figure 3.3.1 at the end of this section. 3.3.2 Patient Transportation/Evacuation 3.3.2.1 Introduction The type of transportation and its frequency of use varies according to the nature of the patient's condition, worksite
location, and NASA Center geography and activities. Evacuation to off site
medical facilities may be by ground or air vehicles as medical situation and distance dictate. 3.3.2.2 Responsibilities a. At each NASA Center, decisions on type of transportation to be made available are made jointly by medical professionals and management officials. Case use is a medical decision. It is also the Center's responsibility to assure the availability and operational readiness of employed transportation/evacuation services,
including type of en route care provided, when called. All such transport must meet local and State requirements. b. The NASA OHP assists NASA Centers in their assessment of need for and decisions on providing these services. The OHP PCO receives reports of incidence of transportation/evacuation of patients
from all NASA Centers and evaluates any anomalous events or unusual trends.
The OHP PCO helps to standardize services across Centers to the extent feasible and practicable and periodically audits their status. 3.3.2.3 Process Description Need for and frequency of patient transportation/evacuation must be assessed by each NASA Center. Method for providing that capability is determined and implemented according to this assessment. Appropriate auditing of quality and readiness of services is conducted by Center officials as well as the OHP. Tracking of Agency statistics and patterns of use for patient transportation/evacuation will be done by the OHP. 3.3.2.4 Flow Diagram The flow diagram for this process is shown in Figure 3.3.2 at the end of this section. 3.3.3 Automatic External Defibrillator Program 3.3.3.1 Introduction The OHP supports the use of Automatic External Defibrillators (AED) at NASA Centers in order to provide a timely response to victims of sudden cardiac arrest caused by ventricular fibrillation. Ventricular fibrillation is a treatable condition and potentially survivable when immediate treatment is provided. The goal of this program is to provide a timely emergency response and treatment for sudden cardiac arrest while ensuring the rapid transfer of the individual into the
community EMS. The ability to respond quickly not only increases the potential survival for the individual, it provides the opportunity for the best possible medical outcome. 3.3.3.2 Responsibilities a. The OHP is responsible for establishing the AED Program Policy and Guidelines and providing support and consultation to the Centers. b. NASA Center Medical Directors have primary responsibility for implementation and oversight of the AED Program. 3.3.3.3 Process Description An AED Program must be implemented at all of the NASA
Centers. It provides the needed emergency response critical
, to a good
outcome. Elements of the process include the following: a. An assessment of Center needs must be completed prior to the planning and implementation stage of the program development. b. An AED Program requires medical direction and oversight by a physician. c. The implementation phase i
ncludes the development of program policy a
, nd procedures, development of emergency res
pons
, e plan, selection responders, and procurement of equipment and supplies. d. All responders must receive initial training and certification in CPR and the use of AED's and periodic retraining. e. The program plan must be integrated into the local community; EMSaston to ensures rapid transfer of care. f. Documentation is a key component of the program and includes incident responses, drills, training records, and equipment maintenance. g. An evaluation of each emergency response and emergency drill includes a critique of the response, feedback to the responders, and modification of emergency response plan when indicated. h. Critical Incident Debriefing is offered to responders after an emergency response when appropriate. i. Program evaluation is an ongoing process to evaluate the effectiveness of training, emergency responses, and event outcomes. j. Access to an AED should be within 3 minutes. 3.3.3.4 References a. Chief Medical Officer's memo "NASA Occupational Health Program Guidelines for Implementing a Center Automatic External Defibrillator Program", July 20, 2000. b. Public Law 106-505, Public Health Improvement Act, Title IV-Cardiac Arrest Survival, November 13, 2000. c. 66 FR 28495, Guidelines for Public Access Defibrillation Programs in Federal Facilities May 23, 2001. 3.3.3.5 Flow Diagram The flow diagram for this process is shown in Figure 3.3.3 at the end of this section. 3.3.4 Treatment Recommendations for Cold Injury Caused by Exposure to Cryogenic Liquids 3.3.4.1 Reserved. a. Reserved. b. Reserved. 3.3.4.2 RReserved. a. Reserved. b. Reserved. 3.3.4.3 Reserved. a. Reserved. b. Reserved. 3.3.4.4 Reserved. a. Reserved. b. Reserved. (1) Reserved. (2) Reserved. c. Reserved. (1) Reserved. (2) Reserved. d. Reserved. (1) Reserved. (2) Reserved. (3) Reserved. 3.3.4.5 Reserved. a. Reserved. (1) Reserved. (a) Reserved. (b) Reserved. (c) Reserved. (d) Reserved. (e) Reserved. (f) Reserved. (2) Reserved. (3) Reserved. (4) Reserved. (5) Reserved. b. Reserved. (1) Reserved. (2) Reserved. (3) Reserved. (4) Reserved. (5) Reserved. (6) Reserved. c. Reserved. (1) Reserved. (2) Reserved. (3) Reserved. d. Reserved.s e. Reserved. f. Reserved. g. Reserved. 3.3.4.6. Reserved. a. Reserved. b. Reserved. c. Reserved. d. Reserved. 3.3.4.7 Reserved. 3.3.5 Medical Response to Nuclear, Biological, and Chemical Warfare Agents 3.3.5.1 Introduction a. Nuclear, Biological and Chemical (NBC) terrorism incident is an intentional act designed to create fear, maim, and kill the public. NBC incidents have the potential to be extremely dangerous. The importance of this subject cannot be overemphasized, and physicians, nurses, and allied professionals will develop a solid understanding of this subject and the medical armamentarium for dealing with these threats. b. Various bacteria, fungi, viruses, rickettsial agents and toxins can be used as potential biological warfare agents. Some of the most commonly mentioned ones are Bacillus anthracis (anthrax), and Yersinia pestis (plague) and can be dispersed in aerosols that can remain suspended in the air for hours. Other possible routes of exposure can obviously be contamination of food and water supplies. c. Chemical warfare agents can consist of various chemical compounds but are broadly divided into 'lethal' agents and 'incapacitating' agents. Some of the commonly mentioned ones are cyanide, mustard gas and nerve agents like acetylcholinesterase inhibitors. 3.3.5.2 Responsibilities a. The medical management of NBC agents is and must
always be a team effort among medical, safety, environmental, security,
facilities and other relevant disciplines which include Federal, State and
local concerns and regulatory agencies, such as the Nuclear Regulatory
Commission, Department of Defense, Federal Emergency Management Agency, CDC, OSHA, and the Environmental Protection Agency. b. The NASA's Emergency Preparedness Managers,
designated by each Center Director, are responsible for compliance with all
policies and regulations applicable to their Centers and for developing plans and resources to meet the needs from an NBC incident. c. NASA Center OHP professionals, including EAP, must develop and implement requirements and strategies for dealing with an NBC
incident, including promoting awareness and maintaining anticipatory readiness, and critical incident debriefing. They also must assure liaisons with Center safety and Emergency Preparedness, and security personnel, as well as other relevant disciplines. This is especially essential with the local police, medical, and public health communities. d. The NASA OHP provides oversight and guidance to
Centers and coordinates intercenter and interagency allocation of resources in incidents involving mass casualties. 3.3.5.3 Process Description a. NASA Centers develop a coordinated, detailed and clear NBC response plan and conduct response exercises and training regularly. b. This plan should include but not be limited to the
following: (1) Potential target sites/ target personnel. (2) Resources needed and their management. (3) Initial response. (4) Early hazard identification. (5) Handling of mass causalities/fatalities. (6) Evacuation, containment, transportation. (7) Triage and treatment. (8) Decontamination, personal protective equipment. (9) Organizations and personnel. (10) Additional responses. (11) Command control, communications, alerts and
notifications. (12) Handling chaos, mass hysteria. (13) Crime scene and evidence preservation. (14) Consider State and Federal support, how to prepare for their arrival, and how to maximally utilize their resources. 3.3.5.4 References a. Medical Management of Biological Casualties
Handbook, U. S. Army Medical Research Institute of Infectious Diseases, Fort Detrick, Maryland. b. Medical Management of Chemical Casualties Handbook, U.S. Army Medical Research Institute of Chemical Defense, Aberdeen Proving Ground, Maryland. 3.3.5.5 Flow Diagram The flow diagram for this process is shown in Figure 3.3.5 at the end of this section. 3.4.1 Infection Control 3.4.1.1 Introduction a. Infection control is an organizationwide program that requires the involvement and commitment of all health care personnel and employees. The program should be a systematic, coordinated, and continuous approach to improving performance, focusing on surveillance, prevention, and control of infections. The scope of the function is broad; it includes activities at the direct patient care level and at the patient care support level to reduce risks of nosocomial/clinic-acquired
infections in patients. Activities are also designed to reduce risks of
transmission of infections among civil service personnel, contractors, health care personnel, students, and visitors. Particular areas of interest for infection control are direct patient care practices, ancillary services such as laboratory, radiology, and rehabilitation, and support services such as linen supply. b. The goal of the infection control program is to identify and reduce the risks of endemic and epidemic nosocomial/clinic-acquired infections in patients and health care workers. Infection control practices must encompass infections that patients may acquire as a result of their care or treatment within the occupational health facility as well as protect health care providers in these settings. To facilitate implementation and assessment of this task, the PCO has developed a checklist that Occupational Health personnel may use to assess their program. The checklist is shown in Appendix F. 3.4.1.2 Responsibilities a. Center Chief Medical Officers/Medical Directors will ensure that an infection control program is established and maintained at their Centers. These officials are responsible for ensuring that adequate resources, including time and training, are available to support the program. b. The infection control program must be the responsibility of at least one person designated by the Center Chief Medical Officer/Medical Director. That individual is known as the Infection Control Officer (ICO) and is responsible for overseeing the program. Specific knowledge and training relevant to infection control will enable the designated person to keep up to date on regulatory changes. 3.4.1.3 Process Description a. The Infection Control Plan plays an integral role in ensuring that the occupational health clinic's infection control program
operates efficiently. The designated ICO establishes, maintains and oversees this process. It is recommended that the ICO establish an Infection Control Committee (ICC). The ICC membership should consist of a physician, a nurse, and any other staff necessary to manage the program effectively. The ICC should coordinate all activities related to the surveillance, prevention, and control of nosocomial infections. b. It is the duty and responsibility of the ICO and/or ICC to develop, implement, and maintain an infection control plan and guidelines that meet the needs of the occupational health facility. The plan should include program goals, surveillance activities, infection control guidelines, infection control training, nosocomial/clinic-acquired infections reporting process, program assessment, performance improvement procedures and program documentation. After the initial plan is developed, it is reviewed every 2 years based on the proceeding year's infection control data by the ICO/ICC. The review should include infectious waste disposal, shelf life of all stored sterile items, reprocessing of nondisposable items, housekeeping contract, linen services, radiology, and laboratory services. c. The infection control guidelines should provide an easy reference to important infection control guidelines and practices. The infection control guidelines and practices address patient care issues such as hand-washing practices, approved antiseptics and disinfectants, sterilization of equipment and disinfecting the clinic, laundry, housekeeping, ventilation, and environmental sampling. There must be a health program for the health care personnel, including immunizations, postexposure protocols and work restrictions/accommodations. The Center Bloodborne Exposure Control Plan and a tuberculosis prevention and control plan are also included as part of the guidelines and practices. The infection control guidelines and practices must be reviewed and updated every 2 years by the ICO/ICC. d. Infection control issues and data, including infections and communicable diseases, immunization status of health care personnel and tuberculosis skin testing conversion data, will be reviewed and summarized on a regular basis by the ICO or ICC to determine if trends are being formed. Appropriate action must be taken on all infection control issues or problems and a process for followup established to ensure effectiveness of the corrective action. To ensure compliance with infection control standards, the ICO and/or the ICC must conduct facility inspections at least annually. e. The ICO must ensure that all health care personnel and facilities comply with applicable Federal, State, and local regulations
including notification of the public health agency when patients or health care personnel are treated for infectious or communicable disease. f. The training of health care personnel is either required by Federal (OSHA) regulations or strongly recommended. The following infection control training is required: (1) Newly assigned health care personnel must receive infection control training within 10 days of placement in clinical environment. (2) Health care personnel must receive infection control training, including OSHA Bloodborne Pathogen, universal precautions and Personal Protective Equipment (PPE), annually. (3) Health care personnel must receive training when
significant regulatory changes occur. (4) Health care personnel providing direct care to patients should receive continuing education on patient care practices to minimize the risk of nosocomial-acquired infections. g. Personnel should have copies of training materials, general, and infection control reference materials available to them. All training and continuing education records must be kept in the health care personnel records for a minimum of 3 years. 3.4.1.4 References a. Occupational Safety and Health Administration (OSHA) Regulations. b. The Centers for Disease Control (CDC) Isolation Precautions and Infection Control in Hospital Personnel. c. Healthcare Infection Control Practices Advisory
Committee (HICPAC). d. Joint Commission on Accreditation of Healthcare
Organizations (JCAHO) Standards. 3.4.1.5 Flow Diagram The flow diagram for this process is shown in Figure 3.4.1 at the end of this section. 3.4.2 Universal Precautions 3.4.2.1 Introduction a. The objective of Universal Precautions is to prevent parenteral, mucous membrane, and skin exposure of health care workers to bloodborne pathogens and potentially infectious materials. Universal precautions are designed to decrease the risk of transmission of bloodborne pathogens. These precautions are intended to supplement rather than replace recommendations for routine infection control, such as using gloves and hand washing. Universal precautions assume that all blood and body fluids must be treated as infectious, regardless of knowledge of the patient's status. Since the risk of transmission from feces, nasal secretions, sputum, sweat, tears, urine, and vomitus is extremely low or nonexistent, precautions do not apply to these fluids unless they contain visible blood. b. Infection control requires the employer and employee to assume that all human blood and specified human body fluids are infectious for hepatitis B, hepatitis C, HIV, and other bloodborne pathogens. The CDC guidelines on universal precautions should be consistently used for all patients and situations in which job exposure to blood or potentially infectious materials may occur and, especially in emergency situations, in which the risk of exposure is increased. 3.4.2.2 Responsibilities a. The Medical Director and Chief Nurse are responsible for ensuring that infection control procedures are followed. b. The NASA Center Medical Director is responsible for ensuring training within the first 10 days of employment for individuals at risk for occupational exposure and annually thereafter. c. Any person who, in the performance of duties, could reasonably be expected to come into contact with blood or other potentially infectious materials is responsible for being successfully trained according to the CDC guidelines and OSHA Standards, Part 1910.1030. It is important for employees who are exposed to blood or body fluids to become familiar with their rights and the employer's obligations. d. The NASA OHP PCO reviews compliance with infection control and universal precaution practices. 3.4.2.3 Process Description Healthcare workers can minimize exposure of their skin or mucous membranes to potentially infective materials with the use of protective barriers. Appropriately designated personal protective barriers such as gloves, gowns, masks, and protective eyewear will be used for all tasks and procedures in which occupational exposure to blood or body fluids is anticipated. If health care workers have exudative lesions or weeping dermatitis, they should refrain from all direct patient care and from handling patient care equipment until the condition resolves. The risk of transmission of bloodborne pathogens can be minimized if health care
workers use the CDC recommendations for universal precautions. 3.4.2.4 References a. OSHA Standard - 29 CFR Part 1910.1030, Occupational Exposure to Bloodborne Pathogens. b. Publication (CPL 2-2.44C) Enforcement Procedures for the Occupational Exposure to Bloodborne Pathogens Standard. c. CDC Morbidity and Mortality Weekly Report, Recommendations for Prevention of HIV Transmission in Healthcare Settings, 1987; 36 (Supplement No. 2S). d. CDC Morbidity and Mortality Weekly Report, Perspectives in Disease Prevention and Health Promotion Update: Universal Precautions for Prevention of Transmission of Human Immunodeficiency Virus, Hepatitis B Virus, and Other Bloodborne Pathogens in Healthcare Settings, 1988; 37(24); 377-388. 3.4.2.5 Flow Diagram The flow diagram for this process is shown in Figure 3.4.2 at the end of this section. 3.4.3 Bloodborne Pathogens 3.4.3.1 Introduction a. On March 6, 1992, the OSHA Bloodborne Pathogen Standard, 29 CFR 1910.1030, took effect. Bloodborne pathogens are pathogenic microorganisms that are present in human blood and can cause disease in humans. Hepatitis B Virus (HBV), Hepatitis C Virus (HCV), and HIV are three of the most serious diseases caused by these pathogens. b. The standard covers any person, including janitorial workers, who can reasonably expect to come in contact with blood or potentially infectious materials as part of their job. Infection control requires the employer and employee to assume that all human blood and specified human body fluids are infectious for HIV, HBV, and other bloodborne pathogens. Where differentiation of body fluids is difficult, all body fluids are to be considered as potentially infectious. c. "Good Samaritan" acts such as assisting a coworker who has a nosebleed would not be covered. 3.4.3.2 Responsibilities a. The Center Medical Director is responsible for
establishing procedures and treatment availability for employees exposed to bloodborne pathogens and for providing training and hepatitis B vaccine to employees with potential occupational exposure. The health care opinion must comply with OSHA regulations. b. NASA Centers are responsible for establishing a written Bloodborne Pathogen (BBP) Exposure Control Plan that identifies workers with occupational exposure to blood and other potentially infectious material. c. NASA Center OHP personnel are responsible for
maintaining confidentiality of testing. d. The NASA OHP periodically reviews Center programs. 3.4.3.3 Process Description a. The BBP Exposure Control Plan must specify a means to protect and train the employees. Through the Exposure Control Plan, on-the-job risks for all employees exposed to blood and body fluids will be reduced. (1) The plan must be accessible to employees and updated annually or when new or revised procedures are implemented. (2) The methods of compliance include universal precautions, engineering and work practice controls, personal protective equipment, housekeeping, and handling of laundry. (3) The standard also covers Hepatitis B vaccination, postexposure evaluation and followup, hazard communication requiring biohazard warning labels, employee training, and recordkeeping. b. NASA Centers must provide any person, who in the
performance of their duties could reasonably be expected to come in contact
with blood or other potentially infectious materials, training according to
OSHA standards and offer the Hepatitis B vaccination within the first 10 days of employment at no cost. c. A declination form must be signed if the employee
chooses not to be vaccinated for hepatitis B. If the employee later chooses to receive the vaccine, the employee will receive it at no cost. Universal precautions MUST be observed. It is important for employees who are exposed to blood or body fluids to become familiar with their rights and the employer's obligations. 3.4.3.4 References a. OSHA Standards - 29 Part CFR 1910.1030 Occupational Exposure to Bloodborne Pathogens. b. Publication (CPL 2-2.44C) Enforcement Procedures for the Occupational Exposure to Bloodborne Pathogens Standard. c. NASA SOLAR Learning and Resources (online Web site at
http://solar.msfc.nasa.gov/solar/delivery/disc/its/private/cgi-bin/certific) 3.4.3.5 Flow Diagram The flow diagram for this process is shown in Figure 3.4.3 at the end of this section. 3.4.4 Immunizations 3.4.4.1 Introduction Many employees are at risk for exposure to and possible transmission of vaccine-preventable diseases because of their work environment or contact with co-workers, patients, or infective material. Maintaining immunity is a vital part of prevention and infection control for workers. The objective is to utilize immunizations to reduce and protect employees from becoming infected through exposure and potential transmission of diseases to other workers. A number of immunizations may be indicated or considered, depending on the risk of exposure to the employee. 3.4.4.2 Responsibilities The NASA Center OHP is responsible for providing immunizations for international travel and health maintenance. The Centers will have educational materials on adult immunizations available for employees. a. All employees should be evaluated for conditions
related to communicable diseases at the time of employment and at periodic
health maintenance exams. This should include medical history, immunization status, and assessment for conditions that may predispose personnel to acquiring or transmitting communicable diseases. It is strongly advised that employees working in the health care field be immunized against hepatitis B, measles, mumps, rubella, influenza, diphtheria, tetanus, and varicella according to the CDC and the Advisory Committee on Immunization Practices (ACIP). At this time, the CDC does not recommend annual tuberculosis testing of health care workers in facilities that are at minimal or very low risk for tuberculosis exposure. b. Since international travel has become more common for employment, employees should be evaluated and educated in advance of travel regarding health risks. Protective immunization guidelines are in the Health Information for International Travel published by the CDC. The CDC also provides a telephone consultation service and FAX-back service. Evaluation and/or testing should be performed as necessary, especially if illness occurred during or after travel. c. An immunization record should be maintained for each employee and reviewed periodically. The record should reflect documented disease and vaccination histories as well as immunizing agents administered during employment. At each immunization encounter, the record should be updated, and the employee encouraged to maintain the record as appropriate. Tetanus and diphtheria status should be reviewed and given, if appropriate, for all employees injured at work. In special circumstances, such as working with lab animals or research, rabies and polio may be suggested. Other considerations important to employees include work restrictions for susceptible workers who are exposed to vaccine-preventable diseases and control of outbreaks in the workplace setting. Exposed workers should be evaluated regarding the circumstances surrounding the exposure, symptoms, and the need for postexposure prophylaxis and treatment. Postexposure work restrictions ranging from limited duty to complete exclusion from duty are appropriate for workers who are not immune to certain vaccine-preventable diseases. 3.4.4.4 References a. 1994 CDC Guidelines for Preventing the Transmission of Mycobacterium Tuberculosis in Healthcare Facilities. b. Centers for Disease Control (CDC) Morbidity and Mortality Weekly Report, Immunization of Healthcare Workers: Recommendation of the Advisory Committee on Immunization Practices (ACIP) and the Hospital Infection Control Practices Advisory Committee (HI
, CPAC), December 26, 1997, Vol. 46, No. RR-18. c. American College of Occupational and Environmental Medicine (ACOEM) Guidelines for Employee Health Services in Healthcare Facilities, V.1.0, 1998. d. Infection Control and Hospital Epidemiology,
, Vol. 20, No. 10, October 1999.
4.1.1 Introduction NASA Centers must comply with applicable OSHA standards promulgated under Section 6 of the OSHA Act of 1970. Exceptions may be made in the case of Alternate or Supplementary Standards, provided those standards were developed in accordance with 29 CFR 1960.17 and 1960.18, respectively. Additionally, NASA may develop specific standards in instances in which a NASA program is intended to clearly exceed the protection offered by compliance to an OSHA Standard. 4.1.2 Responsibilities a. NASA Center OHP personnel are responsible for providing occupational health support that complies with OSHA regulations. If a unique NASA operation requires the need for an alternate or supplementary standard, Center OHP personnel are responsible for identifying that need and proposing and submitting the necessary alternate or supplementary standard with all necessary supporting documentation. If the alternate/supplementary standard is approved, Center OHP personnel must
implement it. b. Center Directors must ensure that their Center OHP complies with all applicable standards and regulations. c. The NASA OHP provides coordination and communication with the NASA Centers and provides technical and subject matter expertise. d. The Designated Agency Safety and Health Officer (DASHO, i.e., the NASA Chief Health and Medical Officer) serves as the Headquarters coordinator for review of alternate and supplementary standards and prepares necessary material for interagency review with the Department of Labor.The DASHO, or designee, also coordinates Agencywide and interagency
reviews and approval and may publish the standards in their final form. 4.1.3 Process Description a. Approval for variances from OSHA standards may be obtained by submitting the proposed alternate or supplementary standards to the DASHO for coordination and approval by the Secretary of Labor. Alternate or supplementary standards are normally adopted as NASA-wide standards. b. Approval for variances from NASA standards will be
accomplished per variance guidelines in NPR 8715.3. NASA Safety Manual, Paragraph 1.20,Safety Risk Acceptance Variance Process. Nonconformance with unique NASA-developed standards for which there are no OSHA standards will only require approval within NASA. c. Alternate Standards: NASA may develop unique alternate health standards provided such standards are approved in accordance with CFR 1960.17.The
DASHO serves as the Headquarters coordinator for review of alternate standards and prepares necessary material for interagency review with the Department of Labor. Requests for alternate standards are coordinated with employees or their representatives and are not approved unless the alternate standard provides equivalent or greater protection for affected employees. d. In developing and submitting alternate standards, NASA shall provide the following information: (1) A statement as to why NASA cannot comply with the OSHA standard or wants to adopt an alternate standard. (2) The proposed alternate standard. (3) An explanation of how the alternate standard provides equivalent or greater protection for the affected employees. (4) A description of interim protective measures employed pending approval of the standard. (5) A written summary of comments, if any, from interested employees, their representatives, or the applicable safety and health committee. e. Supplementary Standards: Unique NASA operations, materials, facilities, equipment, procedures, and practices may require establishment of supplementary health standards. NASA may develop unique supplementary health standards, when no OSHA standard or Voluntary Consensus Standard exists, provided such standards are approved in accordance with CFR 1960.18. When a standard is applicable NASA-wide, the standard is issued as a NASA health standard. The NASA organization proposing the supplementary standard acts as the lead in developing the standard. The DASHO coordinates Agencywide and interagency reviews and approval and may publish the standard in its final form. f. In developing and submitting supplementary standards, NASA shall provide the following information: (1) A statement as to why NASA requires the development of the supplementary standard. (2) The proposed supplementary standard. (3) An explanation of how the supplementary standard provides protection for the affected employees. (4) A description of interim protective measures employed pending approval of the standard. (5) A written summary of comments, if any, from interested employees, their representatives, or the applicable safety and health committee. (6) A Center applying for Voluntary Protection Program (VPP) will inform the DASHO by letter of the Center's intent. 4.1.4 Flow Diagram The flow diagram for this process is shown in Figure at the end of this section. 4.2.1 Introduction The role of industrial hygiene is to anticipate, recognize, evaluate, and control health hazards in the workplace. An effective and proactive mechanism to fill this role and to manage industrial hygiene programs is to employ a systematic and comprehensive approach to exposure assessment. A comprehensive approach results in a thorough understanding of exposures and enables the industrial hygienist to establish priorities for the program. This allows the program to better protect employees and manage exposure related risk. It also places the program in a better position to anticipate and manage unpredictable changes that can occur and enables the more efficient utilization of resources. 4.2.2 Responsibilities a. NASA Center Directors have the responsibility for implementing and operating environmental health programs at their respective Centers in full compliance with the following: (1) NPD 1800.2A, NASA Occupational Health Program, January 16, 2001. (2) NPD 1820.1A, NASA Environmental Health Program, January 16, 2001. (3) NPD 1810.2A, NASA Occupational Medicine Program, January 16, 2001. b. This includes establishing effective organizations to fulfill environmental health requirements using professionally qualified persons and allocating resources for the Environmental Health Program, and ensuring that Center managers and other personnel cooperate with Environmental Health personnel in meeting the requirements of the program and other applicable health policies, standards, and guidelines. c. NASA Center Environmental Health personnel shall perform the following: (1) Establish and direct the exposure assessment program. (2) Assure key competencies. (3) Review assessment conducted by other staff. (4) Direct follow up efforts. (5) Manage the regular monitoring program. (6) Develop a Management of Change system. (7) Use other
knowledgeable personnel and additional experts when required. (8) Evaluate the background systems. (9) Identify personnel requiring surveillance. d. NASA Center Occupational Medicine personnel shall perform the following: (1) Provide health surveillance screening to identify any workers or worker groups who may be at an elevated occupational health risk (2) Work with other functions to assure such workers' exposures have been assessed and control measure decisions made and adopted, as necessary. 4.2.3 Process Description a. Establishment of the Exposure Assessment Strategy (1) Purpose: (2) Outcome (i) Written exposure assessment program. (ii) Defined goals for the exposure assessment program. (iii) Defined roles for the participants. b. Basic Characterization (1) Purpose (2) Outcome c. Exposure Assessment (1) Purpose (2) Outcome (i) List of similar exposure groups. (ii) Each worker is a member of at least one exposure group. d. Defining and Judging Exposure Profiles (1) Purpose (i) To define exposure profiles for the identified exposure groups. (ii) To make judgments about the acceptability of the exposure profiles. (2) Outcome (i) Exposure profile for each exposure group. (ii) Judgment about the acceptability and the uncertainty of the exposure profile for each exposure group. (iii) A determination that, generally, the exposure is either uncertain, unacceptable, or acceptable. Uncertain exposures lead to further information gathering. Unacceptable exposures lead to control of the exposure. Acceptable exposures lead to a programmed reassessment. e. Further Information Gathering (1) Purpose (i) To set priority on exposure groups for further information gathering. (ii) To gather or generate additional qualitative or quantitative information so that exposure groups can be better characterized and/or the risk posed by the exposure better understood. (2) Outcome f. Quantitative Exposure Data If monitoring data are collected, statistical tools can be used to aid in understanding the data and to assist in interpretation and
decisionmaking. The theoretical basis and limitations of the statistical tools used must be understood by the person using them. The goal should
be to ensure as much as possible that measurements are collected randomly and that data reasonably conform to the appropriate distribution. g. Control of Unacceptable Exposures (1) Purpose (i) Prioritize exposure groups with unacceptable exposures for control. (ii) Develop strategy for control. (iii) Protect workers while long-term controls are put in place. (2) Outcome (i) Prioritized control plan. (ii) Short term and long term control options. (iii) Exposures controlled. h. Reassessment (1) Purpose (i) Periodically recharacterize and reassess exposures in order to-- (ii)
Update exposure groups and exposure profiles, (iii)
Identify changes that may influence exposures, (iv)
Identify unacceptable exposures for control, (v)
Identify uncertain exposures for further information gathering. (2) Outcome (i) Prioritized schedule for reevaluation. (ii) Updated basic workplace characterization. (iii) Updated exposure groups and exposure profiles. 4.2.4 Flow Diagram The flow diagram for this process is shown in Figure 4.2 at the end of this section. 4.3.1 Introduction a. The NASA OHP ensures that NASA employees are provided with a healthful workplace environment that is free from harmful levels of exposure to toxic or hazardous chemical, physical, and biological agents. To that end, NASA complies with applicable regulations of other Federal agencies, as well as NASA's health and safety requirements. In the event of conflicting standards or regulatory issuance, the more protective requirements shall be met, until otherwise determined acceptable by an authorized and competent individual (e.g., Certified Industrial
Hygienist). b. NASA follows, at a minimum, all OSHA standards promulgated under Section 6 of the OSHA Act of 1970. These standards include the PEL's for hazardous airborne contaminants identified in 29 CFR 1910 Subpart Z. While the OSHA PEL's carry the weight of law, the majority of them were adopted in 1970 from 1968 consensus values and do not necessarily reflect current scientific data. Additionally, there are currently PEL's established for approximately 400 chemicals. This is a relatively small percentage of the thousands of chemicals that exist. For
these reasons Occupational Exposure Limits (OEL) recommended and established by other acknowledged authorities must be considered in order to fully protect NASA's workforce. OEL's, even those carrying the force of law, are not boundaries between safe and unsafe. Always staying below the limit does not guarantee good health for all workers, nor does going above the limit mean that workers will necessarily experience injurious effects. The proper implementation of OEL's requires people with appropriate training to
continually observe and monitor both the employees and the work environment. 4.3.2 Responsibilities a. NASA Center Directors are responsible for implementing and operating environmental health programs in full compliance with NPD 1820.1, NASA Environmental Health Program, and in conjunction with NPD's 1800.2, NASA Occupational Health Program. This includes establishing effective organizations to fulfill environmental health requirements using professionally qualified persons, allotting resources for the Environmental Health Program, and ensuring that Center managers and other personnel cooperate with Environmental Health personnel in meeting the requirements of the program and other applicable health policies, standards, and guidelines. b. NASA Center Environmental Health managers or their designees are responsible for monitoring the workplace and the workforce and to select the most appropriate and protective OEL's. They are also
responsible for developing and recommending OEL's in the absence of an
existing OEL for a specific chemical. c. The NASA OHP provides technical support to NASA Centers in developing OEL's where none exist. Support may, for example, be in the form of reference materials, literature searches, and consultation with experts. 4.3.3 Process Description a. NASA utilizes OSHA PEL's, Threshold Limit Values (TLV) issued by the American Conference of Governmental Industrial Hygienists or specific NASA Health Standards issued by the OHP, whichever is more stringent b. In the absence of a specific PEL, TLV, or NASA Standard, other sources of OEL's may be utilized. These include the following: (1) National Institute for Occupational Safety & Health Recommended Exposure Limit. (2) American National Standards Institute Standards. (3) National Academy of Science Recommendations. (4) American Industrial Hygiene Association Workplace Environmental Exposure Level. (5) Environmental Protection Agency Recommendations. (6) Deutsche Forschungsgemeinschaft (German Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area) Maximum Allowable Concentration. (7) British Health & Safety Commission and Health & Safety Executive. Occupational Exposure Limits. (8) Chemical Manufacturers. c. When no established OEL exists for a specific chemical, a working OEL may be established after a thorough examination of the data available for that chemical and by following established industrial hygiene exposure limit setting guidelines. While setting OEL's is not an exact science, it does require knowledge, experience, and professional judgment and shall only be undertaken by professionals that possess the appropriate degree of each (e.g., a Certified Industrial Hygienist). This process shall take into account chemical analogy, animal experimentation and
extrapolation, and human experience and epidemiological data. Important data to be considered include but are not limited to the following: (1) Thorough identification of the hazard. (2) Routes of exposure. (3) Chemical specific toxicology data. (4) Physical and chemical properties. (5) Acute toxicity and irritation data. (6) Sensitization studies. (7) Metabolism and pharmacokinetics. (8) Genotoxicity. (9) Reproductive and developmental toxicity. (10) Neurotoxicity. (11) Subacute/subchronic toxicity. (12) Chronic toxicity and oncogenicity. (13) Human use and experience. (14) Scientific references. d. All of the available data shall be thoroughly documented. A written rationale that considers, summarizes, and weighs the importance of all data shall be produced. Additionally, experience and professional judgment shall be applied to weigh all information and apply an appropriate safety factor, based on the strength of the available data before an OEL is recommended. 4.3.4 Flow Diagram The flow diagram for this process is shown in Figure 4.3 at the end of this section. 4.4.1 Introduction To provide environmental health personnel at NASA Centers with the necessary reference material to perform their job, the NASA OHP has provided a primer on air sampling and equipment calibration. This
primer is not intended to take the place of any learned or established methods currently utilized by environmental health personal but as a reference method for those areas that the environmental health professional might not be familiar with or has not worked in recently. 4.4.2 Responsibilities a. NASA Centers are responsible for providing training and equipment necessary to their environmental health personnel so that they may perform their jobs in an effective manner. b. The NASA OHP assesses the quality and consistency of OHP activities and assists with developing resource needs. 4.4.3 Process Description a. The Sampling and Analytical Methods and Equipment Calibration primer follows accepted practice but may use Center individualization. The guide covers such areas as the following: (1) Presurvey activities. (2) Work area walkthroughs. (3) Records review. (4) Sample planning. (5) Performance of the survey. (6) Collection of bulk samples. (7) Ventilation assessment. (8) Shipment of sampling media. (9) Sampling methodology. (10) Sample recording form b. If a sample exceeds standard value, engineering, administrative and personal protective equipment controls will be applied as
appropriate followed by resampling. All results will be recorded and maintained as per OSHA and NASA record keeping requirements. 4.4.4 References a. OHP Web site at http://ohp.nasa.gov for the actual Sampling and Analytical Methods and Equipment Calibration primer and any of the below referenced material for further explanation of analysis or methods. b. Manual of Analytical Methods [Published by: National Institute of Occupational Safety & Health, (NIOSH)]. c. The Occupational Environment - Its Evaluation and Control [Published by: American Industrial Hygiene Association, (AIHA)]. d. Industrial Ventilation "A Manual of Recommended
Practice" latest edition [Published by: American Conference of Governmental Industrial Hygienists, (ACGIH)]. e. The Fundamentals of Industrial Hygiene (Published by: The National Safety Council) f. Air Sampling Instruments, latest edition. (Published by: American Conference of Governmental Industrial Hygienists, ACGIH). 4.4.5 Flow Diagram The flow diagram for this process is shown in Figure 4.4 at the end of this section. 4.5.1 Introduction a. Ergonomics is the science of fitting the job to the worker. When the interface between the job tasks and the worker performing those tasks is not properly considered and effectively designed, Musculoskeletal Disorders (MSD) can result. These disorders are injuries and disorders of the muscles, nerves, tendons, ligaments, joints, cartilage, and spinal discs. They do not include injuries resulting from slips, trips, falls, or similar accidents. Examples of MSD's include carpal tunnel syndrome, tendonitis, sciatica, herniated disc, and low back pain. Work-related MSD's are the most prevalent, most expensive, and most preventable workplace injuries in the country. According to the Occupational Safety and Health Administration-- (1) Work-related MSDs account for more than one third of all occupational injuries and illnesses that are serious enough to result in days away from work. (2) More than 600,000 employees suffer lost-workday MSD's each year. (3) These injuries cost business $15 to $20 billion in workers' compensation costs each year. Total direct costs may run as high as $45 to $60 billion. b. The loss of productivity, the cost of care, will all impact upon mission success. The goal of NASA's Environmental Health Program is to anticipate, recognize, evaluate and control environmental stressors arising from the workplace that may cause sickness, impaired health or well being, or significant discomfort and inefficiency among employees. For this reason, NASA requires usage of methods which provide best practices for employee protection. This approach allows the flexibility that Centers need to address the specific issues and operations that may be unique to each location. It ensures that Centers have a systematic, working process in place so they may take quick and effective action when MSDs occur. 4.5.2. Responsibilities NASA Center Directors are responsible for providing a physically safe and healthy work environment for the Center employees and for implementing and operating environmental health programs. This includes establishing effective organizations to fulfill environmental health programmatic requirements using professionally qualified persons, allocating
resources for the Environmental Health Program, and ensuring that Center managers, supervisors, and other personnel collaborate with Environmental Health personnel in meeting the requirements of the program and other applicable health policies, standards, and guidelines. The implementation of an effective multidisciplinary ergonomics program involving the interaction and cooperation of Medical, Safety, Environmental Health, Facilities, Engineering, and other organizational disciplines should be supported. Policies and practices should be aimed at the identification and prevention of MSDs. 4.5.3 Process Description Each Center's ergonomics program must include at least the following elements: a. Management Leadership and Employee Participation: Management leadership of your ergonomics program must be demonstrated. Employees (and their designated representatives) must have ways to report "MSD signs" and "MSD symptoms;" get responses to reports; and be involved in developing, implementing and evaluating each element of the
program. Policies or practices shall not discourage employees from participating in the program or from reporting MSDs signs or symptoms. Hazard Information and Reporting: b. A method for employees to report MSD signs and symptoms and to get prompt responses must be established. Employee reports of MSD signs and symptoms must be evaluated to determine whether a MSD has occurred. Information to employees that explains how to identify and report MSD signs and symptoms must be periodically provided. c. Job Safety Analysis (JSA) and Process Controls: Problem jobs must be analyzed to identify the ergonomic risk factors that result in MSD hazards. The MSD hazards must be eliminated, reduced to the extent feasible, or materially reduced using an incremental abatement process. d. Training to employees must be provided so they know about MSD hazards and the ergonomics program as well as measures for eliminating or materially reducing the hazards. Training must be provided at no cost to employees, such that they are cognizant of the ergonomics program, MSD hazards, and methods for eliminating MSD hazards. e. Ergonomics program must be reevaluated periodically and identified deficiencies corrected. Metrics that document the efficacy of the ergonomics program shall be maintained and used to improve the ergonomics program and to reduce MSD risks. 4.5.4 References a. NPD 1800.2,NASA Occupational Health Program, dated January 16, 2001. b. NPD 1820.1, NASA Environmental Health Program dated January 16, 2001 c. NPR 8715.1, NASA Occupational Safety and Health Programs. 4.5.5 Flow Diagram The flow diagram for this process is shown in Figure 4.5 at the end of this section. 4.6.1 Introduction Radiological Health, also referred to as Health Physics, is included in the NASA Environmental Health Program. The intent of this program is to exercise centralized control over the procurement, use, storage, transportation, and disposition of ionizing and nonionizing radiation sources in order to limit the exposure of personnel, facilities, and the environment to levels of radiation that are As Low As Reasonably Achievable (ALARA).The goals of the program are to protect the health of the public, astronauts and Pilots, NASA workforce and high value property and equipment so that NASA's mission may be effectively met; and to administer a
program that is in compliance with all applicable Federal, state, and local
regulations. 4.6.2 Responsibilities a. All NASA Centers have the responsibility to ensure that they have a radiation protection program, if applicable, that complies with the above-stated intent and goals. b. The Senior Environmental Health Officer serves as the Agency's Radiation Safety Officer and functions as the liaison between Centers and the OHP. c. The Manager of the NASA OHP has functional management responsibility for the radiological health program which includes coordinating with the Safety and Risk Management Division, the Environmental Management Division, and others as necessary to ensure that applicable responsibilities are met. 4.6.3 Process Description a. The Center Radiological Health program assures compliance with 10 CFR Part 20, 29 CFR 1910.96, 29 CFR 1910.97, and any other applicable regulation. b. The NASA OHP performs the following functions with regard to all occupational aspects of the NASA Radiological Health program: (1) Assesses regulatory compliance with 10 CFR Part 20, 29 CFR 1910.96, 29 CFR 1910.97, and other applicable regulations. (2) Assists Centers with Nuclear Regulatory Commission licensing issues. (3) Provides program oversight. (4) Provides program assessment and auditing. (5) Facilitates intercenter communication. (6) Contributes program advocacy. (7) Provides communication and coordination with the Office of Safety and Mission Assurance, Safety and Risk Management Division (Code QS), and the Environmental Management Division (Code JE) (8) Serves as Agency representative on issues that require interaction and coordination with other Federal agencies and industry groups. c. The NASA Centers maintain Radiological Health Programs (if applicable) that are in compliance with all appropriate Federal, State, and local regulations and that are consistent with the intent of the NASA policy. Each NASA Center communicates and coordinates with the NASA OHP in order that the above functions may be effectively performed. d. Issues concerning the launching of radioactive materials fall under the purview of the Office of Safety and Mission Assurance. Refer to NPR 8715.3, NASA Safety Manual, chapter 5. 4.6.4 References NPR 8715.3, NASA Safety Manual, dated January 24, 2000. 4.6.5 Flow Diagram The flow diagram for this process is shown in Figure 4.6 at the end of this section. 4.7.1 Introduction In conjunction with the Agency's effort to provide its
employees with a safe and healthy workplace, the NASA OHP oversees NASA
Center environmental sanitation policies to ensure that OHP goals are achieved. 4.7.2 Responsibilities a. NASA Centers must develop environmental sanitation
programs that are preventive in nature. They reflect this approach by formally involving their safety and health offices in planning and review of all proposed projects, processes and procedures to eliminate or minimize, in
advance, as many potential health hazards as possible. The Centers ensure that compliance with Agency policies and directives is maintained. b. The OHP PCO assists Centers with developing
environmental sanitation programs and performs audits of existing programs. 4.7.3 Process Description a. The Sanitation Program activities shall be centered around formal design/operational document reviews and both periodic and special surveys. The focus of these activities shall be, but are not limited to, the following: (1) Facility sanitation. (2) Disease vector surveillance. (3) Potable water compliance monitoring. (4) Food service sanitation compliance inspections. (5) Monitoring of chemical toilets. (6) Launch/landing support. (7) Zoogenic problems. b. NASA Centers shall maintain awareness of any developing local public health concern (e.g., West Nile fever from local birds, rodent-borne viruses.) and report any possible concerns to the OHP PCO. Flow Diagram The flow diagram for this process is shown in Figure 4.7 at the end of this section 4.8.1 Introduction a. People in occupations requiring prolonged or unusual time schedules are often subject to extraordinary work stress that has physiological and psychological consequences that can affect health and safety. Consideration of potentially detrimental impacts of unusual or varying worktimes must be given a high priority to prevent worker stress and
undesirable outcomes. b. Reserved. 4.8.2 Responsibilities a. Center Directors and Senior Managers assure
implementation to prevent work excesses and violations. They also provide appropriate means for arbitration of disputes and redress of offenses. b. Center medical staff provide professional consultation to work managers and supervisors regarding requirements for standard and prolonged work schedules and work excesses. c. Center EH Managers assure that potential exposures are appropriately evaluated and that OEL's are appropriately adjusted as necessary from the 8-hour time-weighted average to reflect actual conditions. d. The OHP provides baseline guidance for work
scheduling, assists Centers in their practical implementations, and conducts
periodic audits of work records at Centers. e. The NASA Headquarters OH Office issues relevant policy and directives and provides supporting advocacy and resources. 4.8.3 Process Description a. Reserved.time (1) Reserved. (2) Reserved. (3) Reserved. (4) Reserved. (5) Reserved. (6) Reserved. (7) Reserved. (8) Reserved. (9) Reserved. (10) Reserved. b. Definitions and Determinations (1) Critical job categories are frequently identified in NASA operations. Not all tasks carry equal consequences for degraded performance or failure. Many Personal Reliability Positions (PRP) or Test Sensitive Positions (TSP) fall into the Critical job category. Criticality
encompasses factors such as level of effort, urgency, safety, intrinsic value, success and failure, and consequences. For positions categorized as
"critical," certain specific work-rest criteria will apply. (2) Shift work is defined as work periods requiring well-defined, delimited duty for individuals, in sequential order to assure prolonged or continuous operations. By varying lengths of work periods and carefully scheduling shift changes, worker stress and fatigue can be minimized. A good outcome depends on balancing objective factors such as intensity and duration of effort, vigilance and decisions required, risks to the individual and the process, and also individual preferences. Most people find it easier to rotate work shifts with the earth's rotation-from day to evening to night-rather than counter to it. (3) Circadian rhythms are inherent, periodic,
autonomously running biological cycles normally entrained about the 24-hour, day-night cycle on Earth. Biochemical, endocrinological, immunological, physiological, and psychological processes exert influences causing a variety of system specific amplitudes of generally reproducible peaks and valleys, usually over a 24-hour cycle. (4) Time zone changes-altering or shifting natural bodily rhythms requires considerable time to reach new equilibriums as evidenced in the well-known "jet lag" syndrome. Adaptation to an earlier (east to west) time zone is generally easier than to a later (west to east) time zone. This type of adaptation is similar in rotating work shifts as most people find it easier to shift from day to evening to night periods rather than the opposite direction. Consideration should be given to allowing for adaptation times to avoid critical decisions in a chronobiologically impaired state. Benefits and preferences for scheduled shorter or longer time periods at a given shift remain controversial. In the aggregate, however, circadian rhythms materially affect physical capability, mental alertness and decisionmaking, and overall well being that can predispose to illness or injury-and hence, adversely impact work capacity, quality of performance, and safety. These biological rhythms
cannot be ignored. (5) The calendar year, the week, and the calendar day (which changes at midnight) shall be used for worktime evaluation and maintenance of records. Accurate time records are mandatory in all work considerations. (6) There must be a balance of workload and workforce capability and a balance between work and restorative times-such as sleep or rest, family or nonwork social interactions. On the other hand, a distinction between customary and expected pressures and stresses of meeting deadlines, physical effort, and the rigors of interaction with people and the environment must be clearly made versus those stresses derived from pushing the envelope of psychophysiological endurance and limits. c. Worktimes The following is based upon worktimes used at KSC and Langley Research Center (LaRC), and Commission recommendations for Nuclear Regulatory Commission policy on shift scheduling and overtime at nuclear power plants. (1) For "critical" positions: (i) Level Green-Employee has choice of 5 days of 8 hours per day or 2 consecutive days of 12 hours per day, with a maximum of 60 hrs. per week. (ii) Level Yellow-Where specific job circumstances - "problems during operations"- exist, one should not work more than 16 hours in a day or more than 2 16-hour days in a month with 192 hours maximum. (iii) Level Red-Immediate supervisor approval is needed if above work durations are exceeded beyond 16 hours per day in a week or twice per month, or beyond other green level maximums, to avoid possible adverse health and safety effects. A minimum of 10 hours between shifts is recommended; consideration should be given to a half shift the next day to avoid rotating into another shift cycle. The employee, even with supervisory approval, should not exceed 2260 hours annually. Working
up to 24 hours per day or more than 72 hours per week will require Chief Safety Officer and/or Center Director approval and notification of project and/or line management because of increased safety risk and short-term health effects, and increased risk of impact upon the NASA mission. Since the risks of fatigue, errors, and health effects increase with prolonged work hours, 2300 hours in a year should not be exceeded without Chief Safety Officer and/or Center Director approval; 2500 hours a year should not be exceeded. (2) The ranges of general work positions can be considered as the following, which take into account increasing fatigue with increasing work durations: (i) Level Green-Employee has choice of 5 days of 8 hours per day or 2 consecutive days of 12 hours per day, with a maximum of 60 hours per week. (ii) Level Yellow/Red- Where specific job circumstances - "problem during operation" - exist, one should not work more than 16 hours in a day or more than two 16 hour days in a month with 192 hours maximum and 2260 hours a year. Immediate supervisor approval is needed if above workday durations are exceeded beyond 12 hours per day. Work beyond 16 hours a day would require a Centerwide Declared Emergency (CDE) or Program Declared Emergency (PDE) with Chief Safety Officer and/or Center Director approval. Exceptions or extensions to standard, or "optimal"
lengths of work periods may be required or desirable in particular
circumstances. The traditional "standard" 5-day, 8-hour shift is becoming
frequently replaced with three consecutive, 12-hour shifts - compensated to the worker by more time/days off. Beyond this, further extensions of work intervals may be dictated by an operation/task or preferred by all personnel involved in a given process. Duration and scheduling of off-time intervals must be factored in, and may limit maxima, in any extension of worktime. Principles for assessing the three levels of work scheduling/duration delimited above are given below: (iii) Level Green: The standard, routine, optimal schedule (e.g., 5 8-hour workdays, or shifts, per week), may be sustained by normal, healthy adult workers indefinitely. When the 8-hour period is shifted within the 24-hour day-night cycle, compensatory time must be allowed for circadian rhythms to adapt. Alternatives may include the popular 12-hour shift schedule for not more than three consecutive shifts, and compensatory time for rotation of shifts also applies. (iv) Level Yellow/Red: Work scheduled beyond Level
Green will include occasional, isolated shifts for up to 18 hours with
increased restorative time allowed, especially when high vigilance or important decisions are involved. This situation may also be encountered with long, transmeridional plane travel. 3. Work of such urgent nature or situational circumstances as to require performance essentially at endurance capacity sometimes is required. This may be invoked for life-threatening emergencies, natural disasters, mass casualty accidents, or war. There will always be, however, the red line, an absolute limit to extensions and exceptions for work periods, regardless of the imperative. Such situations demand careful evaluation of the total scenario and prudent scheduling of work periods when pressing workers to and beyond biological limits. Such limits may not be exceeded without consequences to safety and well being of workers and to the integrity of the work process. 4. Overtime may be required because of a problem during operation or because of an extended work process. In either case, overtime shall not exceed the stated guidelines. Unusual circumstances may arise that require deviation from the guidelines. Such deviations shall be authorized by the first-level director. (This authorization must be documented and made available for NASA management inspection.) An extended work process shall not be considered unusual circumstances. 5. NASA Centers may wish to define "very unusual
circumstances" using the categories found in the emergency plans at individual Centers and for specific work processes. 6. Further recommendations concerning routine 12-hour/day shift schedules are as follows: (i) The basic 12-hour/day schedule should be "2-on, 2-off," "3-on, 3 off," or "4-on, 4-off." (ii) Each Center should have the capability to cover
unexpected absences satisfactorily without having individuals work more than 12 hours per day. (iii) The general safety record of the Center should be satisfactory, based on general criteria such as those used in NASA's Langley Research Center. d. Variances Management and unions work together to approve variances to stated guidelines, though advanced written approval is required. In unusual circumstances, a NASA review may be indicated. Managers, supervisors, and other specialists (e.g., Contracting Officer/Contract Technical Monitor, medical representative, Employee Assistance Program counselor, union representative) may participate in such reviews. The objective is to guard against arbitrary interpretation and possible excesses leading to maximum worktime violations. Any violations to maximal worktime requirements shall be reported immediately to the director of the primary organization. Resolution shall be sought at the lowest management level. Final arbitration lies with the Medical Policy Board. e. Recommendations to Minimize Worker Stress and Fatigue Related to Time Factors (1) Define the "standard" work period for all operations and tasks regarding multiple and rotating shifts if required, as well as sleep-rest, breaks, restoration intervals. (2) Clarify responsibilities, work expectations, and
desired outcomes for any process or decision. (3) Minimize negative consequences of shifting worktimes by the following: (a) Having an employee select preferred shifts consistent with mission needs. (b) Considering individual circadian rhythms to insure adequate work and sleep-rest cycles. (c) Allowing adequate time for adaptation and recovery from old to new shift or time zone. (d) Knowing the "criticality" of the work to evaluate risk of physiological and psychological consequences of chronobiological stress. (4) Define "critical job categories" and assure that
employees assigned to these categories understand the full implication with respect to work schedule, load, and irregularities. Educate employees about the work and potential impact including effects on total life style, as workers cannot dissociate worktime from time spent elsewhere. (5) Define "extended" work periods for job categories. (a) Allow "exceptions" to standard work requirements by strict criteria: (b) Need, urgency, benefit. (c) Risks. (d) Prior anticipation noted in position description and by positive work relationships between employee and supervisor. (6) Maintain accurate records of work schedules. (7) Define "violation" of allowable maximum worktime with care to differentiate it from extended work or exceptions, to assure no
misunderstandings. Make consequences of violations unmistakable. (8) Consider salutary and synergistic actions by both
organization and individual. (9) Provide an impartial council to hear and resolve disagreements. f. Adjustment and Application of Occupational Exposure Limits to Unusual or Extended Work Shifts (1) The American Conference of Governmental Industrial
Hygienists (ACGIH) states in the introduction of its publication, Threshold
Limit Values for Chemical Substances and Physical Agents and Biological
Exposure Indices, that Threshold Limit Values (TLV) refer to airborne concentrations of substances and represent conditions under which it is believed that nearly all workers may be exposed day after day without adverse health effects. TLV's expressed as time-weighted averages are based on conventional 8-hour workdays and 40-hour workweeks. These exposure limit values assume a relationship between contaminant accumulation in the body while exposed at work and contaminant elimination from the body while away from work (presuming no additional exposure). Work shifts which extend beyond conventional 8-hour per day/40-hour per week schedules upset the accumulation/elimination balance by providing greater periods of potential exposure and shorter periods of no exposure. The industrial hygienist uses professional judgment to ensure equal protection to workers on unusual shifts. (2) Several models exist which can provide guidance for the adjustment and application of OEL's to unusual shifts. The most commonly referred to being the Brief and Scala model. This model and others are described at length in Patty's Industrial Hygiene and Toxicology. 4.8.4 References a. Rosa, Roger R. and Michael J. Colligan, Shift Work: Health and Performance Effects, Chapter 100, pp 1173-1177. In William N. Rom (Ed.), Environmental and Occupational Medicine (2nd Ed.), Little, Brown and Company, Boston, 1992. b. Scott, Allene Jane (Ed.), Shiftwork-Occupational
Medicine State of the Art Review, Hanley and Belfus, Inc., Philadelphia,
1990. c. Seward, James P., Occupational Stress, Chapter 34, pp 585-600. In J. LaDou (Ed.) Occupational and Environmental Medicine, Appleton & Lange, Stamford, 1997. d. American Conference of Governmental Industrial Hygienists, Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices, 2000. e. Paustenbach, D.J.: Occupational Exposure Limits,
Pharmacokinetics, and Unusual Work Schedules. Patty's Industrial Hygiene and
Toxicology, 3rd Ed. Volume IIIA, The Work Environment, Chap. 7, pp. 222-348. R.L. Harris, L.J. Cralley and L.V. Cralley, Eds. John Wiley and Sons, Inc. New York (1994). f. U.S. Department of Transportation, Publication
DOT-MC-99-129 An Annotated Literature Review Relating to Proposed Revisions to the Hours-of Service Regulation for Commercial Motor Vehicle Drivers, November, 1999. g. Nuclear Regulatory Commission, IE Circular no. 80-82, Nuclear power Plant Staff Work Hours, February 1, 1980. 4.8.5 Flow Diagram The flow diagram for this process is shown in Figure 4.8 at the end of this section. 4.9.1 Introduction a. This section establishes minimum requirements for an Agency-wide Hearing Conservation Policy. Centers, Component Facilities, Headquarters, and JPL are hereafter referred to as "Centers." This section outlines NASA's requirements for preventing noise-induced hearing loss where employees are occupationally exposed to hazardous noise in all occupational settings, including all ground-based operations, all aircraft operations, and all aircraft pilots and crew members. This section does not apply to space flight operations. b. The requirements of the latest revision of 29 CFR 1910.95, Occupational Noise Exposure Hearing Conservation Amendment Final Rule and appendices, and the requirements of the latest revision of 29 CFR 1904.10, Occupational Injury and Illness Recordkeeping and Reporting Requirements, for cases involving occupational hearing loss are incorporated herein by reference and shall be followed unless otherwise specified in this section. c. Where conflicts exist between other NASA health and safety requirements, 29 CFR 1910.95, Occupational Noise Exposure Hearing Conservation Amendment Final Rule and appendices, 29 CFR Part 1904.10, Occupational Injury and Illness Recordkeeping and Reporting Requirements, and this section, the most protective requirements shall apply. d. Centers shall take steps to inform and protect all personnel from potential risks to their hearing that may be encountered in, or derived from, the workplace. e. Centers shall have a written Hearing Conservation Program (HCP) which, at a minimum, addresses the requirements and provisions of this section and requires their contractors to have HCPs in accordance with the NASA FAR Supplement 1852.223-70, Safety and Health. f. Occupational health personnel, trained in sound analysis, noise exposure assessment, hearing protection, audiometric testing, and noise abatement strategies, shall review their Center's HCP, including the hazardous noise training program, for adequacy at least every 3 years and more frequently if program requirements change. g. Centers shall implement a "Buy Quiet and Quiet by Design" program and a system to effectively prioritize noise surveys and noise remediation efforts as part of their HCP and in accordance with the provisions of this section. h. Communication and coordination between and among Center managers, supervisors, employees, engineers, environmental health personnel, and the Medical Director shall be implemented to properly identify, evaluate, and control hazardous noise and to prevent hearing loss caused by exposure to hazardous noise. i. Center Directors and affected Program and Project Managers shall be notified of all operations and equipment not conforming to this section or the Center's HCP. 4.9.2 Responsibilities a. The Designated Agency Safety and Health Official (DASHO) shall provide resources for the development and review of the Agency's Hearing Conservation Policy and for implementation of the Office of the Chief Health and Medical Officer (OCHMO) responsibilities contained therein. b. The Director, Agency Occupational Health Program, shall provide direction for, and approval of, the Agency's Hearing Conservation Policy. c. The Senior Environmental Health Officer shall: (1) Make recommendations and provide advice concerning hearing conservation to the Director, Agency Occupational Health Program, and the DASHO, when requested. (2) Biannually review the adequacy of HCPs at each Center. d. The Chief Engineer and the Assistant Administrator for Infrastructure and Administration shall ensure that "Buy Quiet and Quiet by Design" provisions are integral to the site selection and design of new or modified facilities and equipment. e. The Assistant Administrator for Procurement shall ensure that "Buy Quiet and Quiet by Design" provisions are included in all contracts and in the purchase of new equipment, as appropriate. f. Center Directors, Component Facility Directors, and the Assistant Administrator for Infrastructure and Administration shall ensure the following: (1) Adequate resources are provided to implement Center HCPs. (2) Approved HCPs are implemented at their Centers. g. Facility Managers, design engineers , occupational health personnel, and employers of affected employees shall implement the provisions of their Center's HCP. h. Contracting Officers shall ensure that Center contract requi
, rements incl
, , ude provisio
, ns for writt
, en HCPs in
, a
, cc
, ordance wi
, th the NASA
, FAR Supplement 1852.223-70, Safety and Health. 4.9.3 Process Description a. Definitions
in 29 CFR 1910.95, Appendix I, shall be used in this document unless otherwise defined below. (1) Ability and Risk Evaluations - Evaluations performed for the purpose of determining a worker's ability to perform specific job tasks (ability) and the likelihood of harm, either to the worker or others (risk), in relation to anticipated workplace exposures and job demands. Also includes the processes used to evaluate the ability of individuals to safely perform essential duties, if placed in a noisy work environment, and not pose a health or safety risk to themselves or others. (2) Action Level - An 8-hour time-weighted average of 82 decibels measured on the A-scale, slow response, or equivalently, a dose of 50 percent. Employee exposure at or above the action level triggers enrollment into a hearing conservation program. (3) Administrative Control - Any procedure that limits noise exposure by restricting access to noise areas or by control of exposure times, distance, and/or work practices. (4) Audiogram - A chart, graph, or table resulting from an audiometric test showing an individual's hearing threshold levels as a function of frequency. (5) Audiologist - A professional, specializing in the study and rehabilitation of hearing, who is certified by the American Speech-Language-Hearing Association or licensed by a state board of examiners. (6) Audiometer - An electronic instrument used for measuring hearing threshold levels that conforms to the requirements and specification of the current American National Standard Institute (ANSI) S3.6, Specification for Audiometers standard. (7) Baseline Audiogram - The audiogram against which future audiograms are compared. (8) "Buy Quiet and Quiet by Design" Program - A program that endeavors to achieve long-term reduction of employee noise exposures through purchase and design of equipment with the intention of achieving realistic and achievable noise criteria, which are considered before procurement or design, using criteria based on operational conditions as well as the noise outputs of equipment. The "Buy Quiet and Quiet by Design" approach requires designers and engineers to consider noise emission when purchasing and designing equipment that is expected to generate noise emission levels of concern for hearing conservation (80 dBA and higher). (9) Calibration - A check of proper functioning and stability of an audiometer, sound level meter or octave band analyzer, noise dosimeter, or audiometric test room by various means. Where methods or requirements vary, the methodology or specification that results in the most accurate data collection shall apply. (10) Criterion Sound Level - A sound level of 85 dBA TWA, which is NASA's maximum occupational exposure level. (11) Decibel (dB) - Unit of measure of sound level. (12) Decibel A-weighted (dBA) - A sound level reading in decibels made on the A-weighted network of a Sound Level Meter (SLM) at slow response. (13) Decibels, Peak (dBP) - The highest instantaneous sound level measured. Commonly used to measure impulsive or impact noise. This quantity cannot be measured on the slow response A-weighted scale. (14) De-rating - The process reassigning the manufacturers' values of hearing protectors to more realistic, real-world performance values. (15) Dose - The amount of actual noise exposure relative to the amount of allowable noise exposure and for which 100 percent and above represents noise exposures that are hazardous. (16) Employer - NASA organizations and their associated contractors, to the extent specified in their respective contracts, and other Government agencies, their contractors, and tenants whose primary work is performed at a NASA Center. (17) Engineering Control - Any mechanical device or physical barrier that reduces the sound level at the source of noise generation or along the path of propagation of the noise to the potentially exposed individual. This does not include personal protective equipment such as earmuffs or plugs or administrative controls. (18) Exchange Rate - The increase or decrease in decibels corresponding to twice (or half) the noise dose. When using a 3 dB exchange rate, a dose corresponding to an exposure of 85 dBA TWA represents twice the dose associated with an 82 dBA TWA exposure. (19) Hazardous Noise Area - Any work area where the environmental noise level is at or above 85 dBA, or where the environmental impulse noise level is at or above 140 dB peak C or linear, regardless of duration of exposure or number of impulses. (21) Hertz (Hz) - Unit of measurement of frequency, numerically equal to cycles per second. (22) Impulsive or Impact Noise - Variations in noise levels that involve peaks of intensity that occur at intervals of greater than 1 second. If the noise peaks occur at intervals of 1 second or less, the noise is considered continuous. (23) Medical Pathology - A disorder or disease. (24) Noise - Sound level or sound emission. (25) Noise Dose - A measure of cumulative noise exposure over a stated time period, which takes into account both the intensity of sound and the duration of exposure. (26) Noise Dosimeter - An instrument that integrates a function of sound pressure over a period of time in such a manner that it directly indicates a noise dose. (27) Noise Reduction Rating (NRR) - A noise reduction value, in decibels, averaged across the frequencies from 125 Hz to 8 kHz, computed from laboratory tests of the attenuation of hearing protectors measured under ideal conditions. The NRR, per a 1979-EPA regulation, is required to appear on all devices worn on the head or ear that are sold for purposes of personal noise reduction. See "Derating." (28) Noise Survey - A periodic or event-driven investigation of a hazardous noise, Standard Threshold Shift (STS), or other driving condition for the purposes of determining the noise levels, frequencies, and other sound characteristics as they relate to employee exposure. (29) Occupational Hearing Conservationist (OHC) - A lso known as an industrial audiometric technician. A person who, under the supervision of an audiologist or physician, conducts the practice of hearing conservation, including pure-tone air-conduction hearing testing and other associated duties. (30) Otolaryngologist - A physician specializing in diagnosis and treatment of disorders of the ear, nose, and throat. (31) Representative Exposure - Measurements of an employee's noise dose or 8-hour time-weighted average sound level that is representative of the exposure of other employees exposed to the same noise hazard. (32) Revised Baseline - The most recent audiogram that has established a persistent STS upon retest or a significant improvement. Baseline revisions shall be used as the basis of comparison for future audiograms. Since ears are considered separately when making baseline revisions, it is possible for someone to have baseline audiograms from different years, as well. (33) Significant Improvement - A significant improvement is shown if the average of thresholds at 2000, 3000, and 4000 Hz for either ear shows an improvement of 5 dB or more from the baseline audiogram. (34) Sound Pressure Level (SPL) - 20 times the common logarithm of the ratio of the square of the measured A-weighted sound pressure to the square of the standard reference pressure of 20 micropascals. (35) Sound Level Meter - An instrument for the measurement of sound level. (36) Standard Threshold Shift (STS) - A decline in hearing threshold of 10 dB or more from baseline at 2000, 3000, and 4000 Hz (average) in either ear. (37) Time-Weighted Average (TWA) Sound Level - That sound level which, if constant over an 8-hour exposure, would result in the same noise dose as is measured. (38) Work Role Position - Any job or position at a Center that does not change appreciably when a contract is awarded to a new contractor and the same employee of the former employer occupies the position. b. Written HCP: (1) All Centers shall develop and maintain a written HCP to implement the requirements of this section. (2) At a minimum, the HCPs shall include provisions for: (i) Specifying the individual responsibilities of Facilities Managers, Design Engineers , Occupational Health Personnel , Supervisors, and Employees. (ii) Assuring that noisy areas are surveyed to determine if they are hazardous noise areas. (iii) Affirming the criterion sound level and exchange rate. (iv) Evaluating and maintaining the HCP's effectiveness. (v) Implementing "Buy Quiet and Quiet by Design" Programs. (vi) Exposure monitoring. (vii) Medical surveillance (audiometric monitoring). (viii) Notification and coordination between employees, management and occupational health personnel of noise exposure and dosimetry monitoring and survey results, operational and design plan review results, the addition of new equipment or new operations, and any work-related STS. (ix) Audiometric testing, review, and medical follow-up. (x) Selection, use, cleaning, and inspection of hearing protectors. (xi) Training and certification for employees and supervisors exposed at or above the action level. (xii) Certification of occupational hearing conservationists. (xiii) Recordkeeping and access to information. (xiv) Policy documentation. (xv) Noise control requirements and strategies. (xvi) Effective implementation of engineering, operational, and administrative controls. (xvii) Appropriate corrective actions for employees who violate requirements of this section, the Center's HCP requirements, or 29 CFR 1910.95, "Occupational Noise Exposure Hearing Conservation Amendment Final Rule," and appendices. c. HCP Participation: Whenever an employee is occupationally exposed to noise equal to or exceeding the action level (an 8-hour time-weighted average sound level [TWA] of 82 decibels measured on the A scale [slow response] or, equivalently, a dose of 50 percent for 30 days or more per year), the Center or Facility shall administer a continuing, effective hearing conservation program in conformance with the requirements of this section and the affected employees included in the program. Exposures shall be computed without regard to any attenuation provided by the use of personal protective equipment. d. Noise Exposure Limits: (1) NASA's allowable noise exposure limit is the equivalent to an 85 dBA, 8-hour TWA exposure using a 3 dB exchange rate. Table 1 contains noise exposure levels and durations that are equivalent to this limit as calculated by the following formula where L stands for exposure level and T for duration: T (min) = 480/2 (L-85)/3 Exposures exceeding the equivalent exposures in Table 1 shall be controlled, reduced, or eliminated through a hierarchical combination of engineering controls, administrative controls, and hearing protection devices. Table 1 Noise Exposure Limits Hours Minutes Seconds 80 25 24 0 81 20 10 0 82 16 0 0 83 12 42 0 84 10 5 0 85 8 0 0 86 6 21 0 87 5 2 0 88 4 0 0 89 3 10 0 90 2 31 0 91 2 0 0 92 1 35 0 93 1 16 0 94 1 0 0 95 0 47 37 96 0 37 48 97 0 30 0 98 0 23 49 99 0 18 59 100 0 15 0 (2) Noise dose shall include all impact/impulse noise measured up to and including 140 dB peak. (3) The action level is 82 dBA 8 hour TWA. (4) All personnel who enter designated areas or who perform tasks where exposure to noise is greater than or equal to 82 dBA regardless of the duration of exposure shall be provided with personal hearing protection. All personnel who enter designated hazardous noise areas or who perform tasks where exposure to noise is greater than or equal to 85 dBA or 140 dB peak, regardless of the duration of exposure or number of impulses, shall be provided with and shall be required to wear personal hearing protection. e. "Buy Quiet and Quiet by Design" Programs shall: (i) Meet realistic and achievable baseline noise criteria and optimize noise emission criteria based on individual and specific operational and site conditions. (ii) Encompass design and development, or selection and purchase, of a broad variety of fixed and portable equipment purchased for use by Centers, including equipment purchased by contractors, to minimize noise-exposure hazards to personnel. (iii) Encompass equipment expected to produce noise which is approaching hearing conservation levels of 80 dBA and higher under a variety of site and operational considerations. (iv) Identify noise emission and control requirements for equipment procurement specifications and design. (v) Contain provisions for "Buy Quiet and Quiet by Design" program support, promotion, training, and management sponsorship. (vi) Be individualized to meet the Center's specific needs, configuration, and other relevant factors. (vii) Not apply to specialized research project items or flight hardware, which may be expected to produce large amounts of noise. f. Engineering Controls: (1) Engineering controls shall be the first and primary means of controlling hazardous noise. The feasibility and cost of engineering controls may be considered when making decisions about these controls. (2) Engineered noise controls should attempt to reduce noise emissions (measured at operator position or equivalent) to below 85 dBA. (3) Facility plans shall be reviewed to assess the adequacy of precautions that are planned and/or undertaken to control noise exposures. (4) Engineering projects, drawings, and operational plans, including noise control measures, shall be coordinated with affected management organizations and occupational health personnel in the early stages of the design and/or planning process and prior to contract award and/or authority to proceed. (5) Organizations responsible for introducing changes to facilities, operations, or procedures shall notify occupational health personnel of: (i) Any changes in operations or equipment that increase noise levels. (ii) Any new, uncontrolled, or previously unidentified areas, operations, or equipment that may produce hazardous noise or may not comply with the requirements of this section. g. Administrative Controls: (1) If engineering controls fail to reduce sound levels within the requirements specified in this section, administrative controls shall be utilized. Examples of administrative controls include access restrictions and time limitations in the hazardous noise area. (2) The distance between the employee and the hazardous noise source shall be maximized to the extent practical. (3) Hazardous Noise Area Identification shall be implemented as follows: (i) Areas determined to be hazardous noise areas shall be identified by posting with signs that conform to 29 CFR 1910.145 - Specifications for accident prevention signs and tags requirements. (ii) Signs shall clearly indicate the presence of hazardous noise and state the requirement to wear hearing protection. The signs shall be posted at the entrance(s) to or the periphery of hazardous noise area(s). (iii) Decals or placards with similar statements shall be affixed to power tools and machines that produce hazardous noise levels, and cautions signs shall be posted in areas where hazardous noise-producing tools and machines are used. h. Personal Hearing Protection Devices (HPDs): (1) If both engineering and administrative controls fail to reduce sound levels to 85 dBA TWA or below, p ersonal hearing protection shall be used to bring exposure levels to acceptable levels . (2) HPDs shall be made available for use in sound levels at or above 82 dBA. HPDs shall be worn by employees when they are exposed to noise levels in excess of 85 dBA, independent of duration of exposure. (3) Earplugs shall be for the exclusive use of each employee and shall not be traded or shared. (4) HPDs shall attenuate employee noise exposure to an 8-hour TWA of 85 dBA or less. For those with STS, HPDs shall attenuate exposure to an 8-hour TWA of the 82 dBA or less. (5) The following derating criteria shall apply for all types of HPDs, where "NRR" is the manufacturer's Noise Reduction Rating: Required NRR = [(LA - 85) x 2] + 7, where L A is the measured ambient sound level to which the employee is exposed. (6) The adequacy of HPD attenuation shall be reevaluated whenever employee noise exposures increase. (7) Special hearing-protective equipment, such as sound-suppression or noise-cancellation communication headsets, shall be regularly inspected if they are used in hazardous noise areas. (8) Sound-suppression and noise-cancellation headsets that have been damaged, altered, or modified in any way that affect the attenuation characteristics shall not be used. (9) Where sound-suppression and noise-cancellation headsets are not permanently issued to individuals, such equipment shall be cleaned and sanitized before re-issuance. i. Exposure Monitoring: (1) Noisy areas shall be surveyed to determine if they are hazardous noise areas. (2) Measurement of potentially hazardous sound levels shall be conducted when any information, observation, or calculation indicates that an employee may be exposed to noise at or above the action level. This includes, but is not limited to, times where there is a need to document representative noise exposures, where employees complain of excessive noise, or where it is difficult to understand a normal conversation when the speaker and listener face each other at a distance of 3 feet. (3) In determining TWA exposures, all continuous, intermittent, and impulsive sound levels, from 80 dBA to 140 dBA, shall be integrated into the noise measurements. (4) Octave band analysis shall be conducted, when required, to establish the characteristics of the noise source and to help determine appropriate abatement techniques. (5) When a noise survey is performed, it shall determine the presence of compounding hearing-related circumstances present in the environment (e.g., certain solvents, heavy metals, carbon monoxide, heat, and vibration) to ensure proper mitigation. (6) Noise surveys shall also be conducted whenever any changes to facilities, equipment, work practices, procedures, or noise-control measures alter potential noise-exposures. A review of hazardous noise sources and controls, employee exposures, and work practices and procedures shall be conducted for changed conditions whenever an employee experiences an STS. (7) When a noise survey shows that any employee or group of employees may be exposed to noise at or above 82 dBA 8-hr TWA, noise dosimetry monitoring will be conducted to determine the noise dose of the exposed employee and the representative exposure of similarly exposed employees and to determine appropriate noise abatement techniques. (8) All noise surveys and personal noise dosimetry monitoring conducted shall be consistent with 29 CFR 1910.95 requirements, unless otherwise specified in this section. (9) Operational plans shall be reviewed to assess the adequacy of precautions that are planned and/or implemented to control noise exposures. (10) Baseline surveys of each new or changed operation, job, or procedure, having the potential of creating hazardous noise, shall be conducted. (11) New equipment, operations, jobs, or procedures, with the potential for creating hazardous noise, shall be evaluated with regard to noise emissions prior to operational start-up. (12) Employees and/or their representatives shall be provided an opportunity to observe noise dosimetry and area monitoring activities. (13) Affected employees shall be notified in writing of the results of noise dosimetry monitoring. (14) Employers of affected employees and the appropriate occupational health program managers shall be notified when noise measurement data indicate that noise exposures equal or exceed the limitations of Table 1 and the action level. Written reports of the hazardous noise surveys that identify all survey observations, findings, and conclusions shall also be provided to affected employees. (15) Randomly selected hazardous noise areas shall be surveyed and documented each year to assure program effectiveness. j. Sound-Measuring Equipment: (1) An instrument used to measure workers' noise exposures shall be field-calibrated prior to each use and shall be checked periodically, at least annually, by its manufacturer, a representative of its manufacturer, or an approved laboratory. (2) Sound-level meters used to measure workers' noise exposures shall be set at "slow" response and A-weighting. k. Audiometric Test Equipment: (1) Audiometric test equipment shall be calibrated to meet the requirements specified in the latest revision of ANSI S3.6, Specification for Audiometers. (2) Ambient noise levels in audiometric test rooms and booths shall meet the specifications in the latest version of ANSI S3.1, Maximum Permissible Ambient Noise Levels for Audiometric Test Rooms. l. Medical Surveillance: (1) Audiometric Examination: (i) Employees enrolled in an HCP shall receive medical surveillance as part of the HCP. (ii) Employees receiving medical surveillance shall undergo a baseline audiometric examination before beginning work assignments in hazardous noise areas. If it is not possible to obtain the baseline prior to noise assignment, then employees shall undergo a baseline audiometric examination within 30 days of initial exposure to hazardous noise . During this 30-day period, employees shall wear personal HPDs, which reduce their exposure to 82 dBA TWA or below. When it is discovered that personnel have already been assigned to a position that may expose them to hazardous noise but have not yet had an audiometric examination, audiometry shall be conducted within 30 days of the discovery, and employees shall wear personal hearing protection that reduces their exposure to 82 dBA TWA or below. (iii) Audiometric examinations shall include an audiogram, an otoscopic examination by an audiologist, physician, or Council on Accreditation for Occupational Hearing Conservation (CAOHC)-certified occupational hearing conservationist, unless otherwise appropriately trained, to determine any existing medical pathology of the ear, and an update to their medical history (occupational and personal) to document past noise exposure and other otopathological factors. (iv) The employee shall have no apparent or suspected ear, nose, or throat problems that might compromise the validity of the audiogram. When an employee has an acute disease that may compromise the validity of the test, the audiogram shall be delayed until the condition has abated.
,
(v) At the time
,of the audiometric examinatio
, n, exposure history s
, hall be collected to include ototoxic medications and exposure, to ototoxic substances . (vi) If during a medical evaluation the employee is identified as potentially unable to perform the job safely, the employee and employer shall receive a written notification of the requirement to perform an Ability and Risk Evaluation. The written notification shall include results of the medical and work history with special emphasis on the association of any health conditions that may impair ability to safely perform the work expected in the position held (hear commands or signals) or the ability to wear appropriate personal hearing protection equipment in a hazardous noise area. (vii) Personnel suffering from acute diseases of the ear shall not be placed in hazardous noise areas until the condition has abated, particularly if such diseases preclude the wearing of hearing protectors. (viii) Centers shall take all possible measures to assure that employees who have participated in the HCP medical surveillance program receive a final audiometric examination prior to termination of employment, transfer to duties not involving noise exposures, transfer to another installation, or retirement. An annual audiogram, if completed within 6 months of the termination, transfer, or retirement date, may be substituted for the final audiogram. (ix) When employees at a Center retain their "work role position" but change employers due to contract award to a new employer, all medical records applicable to hearing conservation shall follow them to their new employer , including their current baseline threshold. (2) Audiometric testing: (i) Audiometric testing shall be performed upon initial assignment, and annually thereafter, in accordance with 29 CFR 1910.95 Sections (g) and (h). (ii) Audiometric testing shall be performed by, or overseen by, an audiologist or a physician knowledgeable in hearing conservation. (iii) Personnel who conduct audiometric testing shall be familiar with the provisions of this section, and shall be certified by the CAOHC, or be an audiologist. (iv) All baseline audiograms shall be preceded by a period of at least 14 hours during which there is no known exposure to noise above 82 dBA TWA, on or off the job. Hearing protectors that lower workplace noise to the equivalent of 82 dBA TWA, using the appropriate noise-reduction rating, may be used as a substitute for the requirement that baseline audiograms be preceded by 14 hours without exposure to workplace noise. (v) If the answer to ALL of the questions below is "yes," the STS shall be logged as an OSHA-recordable event in accordance with 29 CFR 1904.10: a) Did an annual age-corrected audiogram reveal an STS (10 dB shift or greater, averaging 2k, 3k, and 4k Hz) relative to the baseline audiogram in one or both ears? (Age correction permitted.) b) Is the employee's uncorrected hearing level (averaging 2k, 3k, and 4k Hz) 25 dB or greater above audiometric zero in the same ear as the STS? (Age correction NOT permitted.) c) Is the hearing work related in the opinion of the medical and supervisory personnel? (vi) If during a baseline audiogram the employee has a hearing profile equal to or worse than that listed in Table 2 below, the employee and employer shall receive a written notification of the requirement to perform an Ability and Risk Evaluation. The written notification shall include results of the medical and work history with special emphasis on the association of any health conditions that may impair ability to safely perform the work expected in the position held (hear commands or signals) or the ability to wear appropriate personal hearing protection equipment in a hazardous noise area. Table 2 Frequency (Hz) 500 1000 2000 3000 4000 6000 Hearing Threshold Level (dB) 25 25 25 35 45 45 (3) Threshold Shifts: (i) The STS may be computed using the age corrections described in OSHA 29 CFR 1910.95, Appendix F. (ii) Each employee's annual audiogram shall be compared to his/her baseline audiogram to determine if the audiogram is valid and to determine if an STS has occurred. (iii) The baseline of each ear shall be separately tracked. (iv) A physician, audiologist, or CAOHC-certified occupational hearing conservationist shall perform the hearing test and the comparison. (v) If an STS is identified and a confirmation audiogram is not performed within 30 days, the STS shall become a confirmed STS by default. (vi) If the identified STS is followed by a confirmation audiogram and the confirmation audiogram does not confirm the STS, this second audiogram replaces the first one that suggested the STS. (vii) If the identified STS is followed by a positive confirmation audiogram, the better of the two shall become the confirmed STS. (viii) A physician or audiologist with hearing conservation experience shall review problem audiograms, including those showing an STS (either by confirmation with 30 days or by default) and shall determine whether there is a need for further evaluation. During the confirmation audiogram, the employee shall be examined by a physician, audiologist, or CAOHC-certified occupational health nurse for proper HPD fit. (ix) When further evaluation is warranted, the employee shall be referred to an otolaryngologist or other qualified physician, or to an audiologist for further medical evaluation. See Section 4.9.3.l.(4), Referrals. (x) A new baseline reference audiogram shall replace the original or previous baseline audiogram (in separate ears and not both ears, unless both ears meet criteria listed below) when: a) The reviewing CAOHC-certified hearing conservationist determines that an STS is persistent on a retest (conducted no sooner than 6 months later). Unless an audiologist or physician determines and documents reasons for not revising the baseline, the baseline shall be revised to the lower (more sensitive) value for the average. Employees assigned a new baseline audiogram, as a result of an STS, shall receive an audiometric re-evaluation 6 months after this assignment to determine if a further STS has occurred. b) A "significant improvement" is shown if the average of thresholds at 2000, 3000, and 4000 Hz for either ear shows an improvement of 5 dB or more from the baseline and the improvement is persistent in the next test. The baseline shall be revised to the lower (more sensitive) value for the average unless a physician or audiologist determines and documents reasons for not revising. Age corrections shall not be used when determining "improvement." (xi) The employee, employer, and environmental health staff shall be notified of an STS in writing within 21 days of the determination of the STS. (xii) Based on the best available information, the employer of an employee with an STS shall determine, in coordination with the physician or physician representative and the employer's health and safety representative, if the noise-induced STS is the result of occupational noise exposure. (xiii) Where it has been determined that an employee has experienced an STS as a result of an occupational noise exposure: a) The employee shall be examined by a physician or an audiologist for proper HPD fit. b) HPDs shall be reevaluated for effectiveness and the employee shall be refitted as necessary with HPDs offering a greater sound attenuation. c) The work environment(s) shall be investigated to determine if work practices or changes in equipment or procedures have increased the noise hazard and abatement actions shall be instituted, as necessary. d) The work-related hearing loss shall be reported to the Center's mishap reporting system. e) The employee shall be trained or retrained on the hazardous effects of noise and the need to use hearing protection. f) Engineering controls shall be employed to reduce the potential for exposure to action level. g) Administrative and work practices shall be reevaluated for effectiveness. h) The employee shall be rechecked or refitted with hearing protectors, offering greater sound attenuation, if needed. i) The employee's management and responsible safety and health office will be notified of the occurrence of an STS or other work-related hearing loss. (xiv) When an OSHA-recordable STS has occurred, the employer shall record the condition as a hearing loss on the OSHA 300 Log and maintain the record in accordance with 29 CFR 1904, Recording and Reporting Occupational Injuries and Illnesses. (xv) The Medical Director shall determine if reassignment to work in a low noise area is indicated to prevent further hearing impairment and shall advise the employer accordingly. (xvi) The employer shall have ultimate authority and responsibility for employee reassignment. (xvii) Where the same employee experiences any subsequent work-related STS as a result of occupational noise exposure, the work environment(s) shall be reevaluated. If the employee continues to work in the hazardous noise area(s), engineering and/or administrative controls shall be employed that reduce that employee's noise exposure to no more than 50 percent of what was previously allowed for that employee. (4) Referrals: (i) Employees shall be referred to an otolaryngologist or other physician knowledgeable in hearing conservation or to an audiologist based on the criteria in this section. (ii) Where further medical testing or referrals are needed, the employee shall be notified of the reason for the testing or need for referral. (iii) When the examining physician refers an employee to a specialist, communication of relevant medical data shall be provided to the specialist. (iv) The following criteria are based upon the American Academy of Otolaryngology-Head and Neck Surgery referral criteria and shall be used for referral to a qualified physician or otolaryngologist for more comprehensive testing and/or treatment: a) Average hearing level at 500, 1000, 2000, and 3000 Hz greater than 25 dB HTL in either ear (Baseline Audiogram). b) Difference in average hearing threshold level between the better and poorer ears of more than 15 dB HTL at 500, 1000, and 2000 Hz (Baseline Audiogram). c) Change for the worse in average hearing level in either ear compared to the baseline audiogram of more than 15 dB at 500, 1000, and 2000 Hz or more than 20 dB at 3000, 4000, and 6000 Hz (Periodic Audiograms). d) Variable or inconsistent responses or unusual hearing loss curves (Periodic Audiograms). e) History of ear pain; drainage; dizziness; severe, persistent tinnitus; sudden, fluctuating or rapidly progressive hearing loss; or a feeling of fullness or discomfort in one or both ears within the preceding 12 months (Any Audiogram). f) Earwax accumulation sufficient to completely obstruct the view of the eardrum with otoscopy or foreign body in the ear canal. g) The employee has failed any of the above criteria and has ear pain; drainage; dizziness; or severe, persistent tinnitus (Any Audiogram). h) When an employee suspects that a medical pathology of the ear is caused or aggravated by the wearing of hearing protectors (Any Audiogram). m. Impairment The latest edition of the American Medical Association Guides to the Evaluation of Permanent Impairment shall be used as a guideline in determining hearing impairment. n. HCP/Hazardous Noise Training: (1) Each occupational hearing conservationist shall receive CAOHC certification training. A CAOHC refresher course shall be taken every 5 years, at a minimum. (2) Occupational health personnel who conduct assessments shall receive initial training in their Center's hearing conservation program and in noise exposure hazard training. (3) Employees enrolled in an HCP and their supervisors shall receive annual training in the hazards of noise exposure. (4) Annual training in the hazards of noise exposure shall include, at a minimum: (i) An overview or review of this section. (ii) An overview or review of the 29 CFR 1910.95, their Center's and employer's HCP, and this section. (iii) The effects of hazardous noise and ototoxic substances on hearing (including permanent hearing loss). (iv) Identification of the hazardous noise sources in the employee's work areas. (v) Factors that may contribute to hearing loss. (vi) Noise-control principles. (vii) An explanation of the audiometric testing procedure and the purpose of audiometric testing. (viii) The employee's role and responsibilities in the HCP. (ix) The purpose of HPDs including: a) The advantages, disadvantages, and attenuation characteristics of various types of HPDs. b) Instructions on selection, fitting, use, and care of HPDs. c) The recommendation that employees use hearing protection whenever they are exposed to hazardous noise during off-duty activities (e.g., lawn mowing, use of firearms). o. Other Considerations: (1) Recordkeeping. (i) Accurate HCP records shall be maintained as specified in the applicable records retention schedules in NPR 1441.1 and 29 CFR 1910.95 (m), Recordkeeping. Records kept shall include: a) The Center's written HCP and subsequent revisions. b) A comprehensive registry of all personnel placed in the HCP. * c) Audiometric tests and records. * d) Background sound pressure levels of audiometric test rooms. e) Data and information concerning repair of audiometers. f) Hazardous noise areas and noise levels recorded in those areas. g) Survey and dosimetry results and recommendations. * h) Data and information concerning calibration and repair of sound-measuring equipment. i) The employee's most recent noise-exposure assessment. j) Special noise studies. k) Remedial actions recommended/taken. l) Engineering controls installed. m) Design operational and review results. n) Training. o) Hearing protector selection. p) Documentation of other official HCP-related activities. (ii) Items above marked with an asterisk ( * ) shall be maintained for at least 30 years. (iii) Audiometric test records shall include, as a minimum: a) Hearing threshold levels at 500, 1000, 2000, 3000, 4000, 6000, and 8000 Hz. b) The audiometric reference level to which the audiometer was calibrated at the time of testing. c) The date of the audiogram. d) The name, employee number, and job classification of the employee tested. e) The examiner's name and position. f) The date of the last calibration of the audiometer. (iv) Consistent with the requirements of the Privacy Act and the restrictions in the "Annual Notice and Amendment to Systems of Records," published in the Federal Register, copies of this section, 29 CFR 1910.95, "Occupational Noise Exposure Hearing Conservation Amendment Final Rule" and appendices, and any other records required by this section, shall be provided upon request to: a) Employees and former employees and their representatives. b) Representatives of the U.S. Department of Labor. c) The National Institute for Occupational Safety and Health (NIOSH). d) Occupational Health Program personnel. (v) Audiograms and noise-exposure records shall be maintained as a permanent part of an employee's medical records. (vi) When noise-exposure-measurement records are representative of the exposures of other individuals participating in the Hearing Conservation Program, and to the extent allowable by the Privacy Act, the range of noise levels and the average noise doses shall be made a permanent part of the medical records of those other individuals. 4.9.4 Authorities a. 42 U.S.C. º 2473 (c) (1), Section 203 (c) (1) of the National Aeronautics and Space Act of 1958, as amended. b. 5 U.S.C. º 552a, the Privacy Act of 1974, as amended. c. 29 U.S.C. º 668, Occupational Safety and Health; Programs of Federal Agencies. d. 29 CFR Part 1904.10, Occupational Injury and Illness Recordkeeping and Reporting Requirements. e. 29 CFR Part 1910.95, Occupational Noise Exposure Hearing Conservation Amendment Final Rule. f. 29 CFR Part 1960, Basic Program Elements for Federal Employee Occupational Safety and Health Programs and Related Matters. g. 29 CFR Part 1910.1020, Access to Employee Exposure and Medical Records. h. 48 CFR Part 1823, Environment, Energy and Water Efficiency, Renewable Energy Technologies, Occupational Safety, and Drug-Free Workplace. i. EO 12196, Occupational Safety and Health Programs for Federal Employees, dated February 26, 1980.
4.9.5 References a. NPD 1800.2B, NASA Occupational Health Program. b. NPD 1810.2B, NASA Occupational Medicine Program. c. NPD 1820.1B, NASA Environmental Health Program. d. NPD 1840.1B, NASA Workers' Compensation Program. e. NPR 1441.1D, NASA Records Retention Schedules. f. NPR 8715.1, NASA Occupational Safety and Health Programs. g. NPR 8621.1B, NASA Procedural Requirements for Mishap and Close Call Reporting, Investigating, and Recordkeeping. h. ANSI S1.4, Latest revision, Specification for Sound-Level Meters. i. ANSI S1.11, Latest revision, American National Standard Specification for Octave-Band and Fractional-Octave-Band Analog and Digital Filters. j. ANSI S1.25, Latest revision, Specification of Personal Noise Dosimeters. k. ANSI S3.1, Latest revision, Maximum Permissible Ambient Noise Levels for Audiometric Test Rooms. l. ANSI S3.6, Latest revision, Specification for Audiometers. m. ANSI S3.20, Latest revision, Bioacoustical Terminology. n. ANSI S3.44, Latest revision, Determination of Occupational Noise Exposure and Estimation of Noise-Induced Hearing Impairment. o. ANSI S12.6, Latest revision, Methods for Measuring the Real-Ear Attenuation of Hearing Protectors. p. ANSI S12.19, Latest revision, Measurement of Occupational Noise Exposure. q. NHCA Professional Guide for Audiometric Baseline Revision. (Latest revision.) r. NIOSH Criteria for a Recommended Standard, 1998. s. American Medical Association, Guides to the Evaluation of Permanent Impairment. t. Suter, A. (2002), Hearing Conservation Manual, Council for Accreditation in Occupational Hearing Conservation, fourth edition. 4.10 Food Safety 4.10.1.0 Introduction This program establishes a preventive approach to food safety. It encompasses planning and reviews of all proposed projects, processes, and procedures to mitigate potential food safety hazards. The primary objectives of this section are to adopt the Food and Drug Administration (FDA) Food Code as the minimum standard for food service operations at NASA facilities and to provide a NASA policy that promotes good retail food practices and active managerial control of the risk factors most commonly associated with foodborne illness. This shall be accomplished through the implementation of Hazard Analysis Critical Control Point (HACCP) principles, risk-based inspections and controls, and FDA-recommended program standards. Where conflicts between the latest FDA Food Code, this section of NPR 1800.1, and any additional state or local "food code" requirements exist, whichever is most stringent shall be applied. Centers shall coordinate questionable conflicts between the latest FDA Food Code and this section of NPR 1800.1 with the Senior Environmental Health Officer (SEHO) and shall coordinate questionable conflicts between either the FDA Food Code or this section of the NPR with state and local ?food code? with the local authorities. This section does not apply to spaceflight operations. 4.10.2.0 O rganization Overall authority for Agency food safety is delegated to the NASA SEHO by the NASA Director of Occupational Health. 4.10.3.0 R esponsibilities 4.10.3.1 The NASA SEHO shall be responsible for managing, evaluating, and reviewing the NASA Food Safety Program to ensure compliance with regulatory and Agency requirements. Specifically, the SEHO shall be responsible for: a. Acting as the functional representative of the Director of Occupational Health on food safety matters. b. Providing technical guidance to Centers' food service organizations on related food safety matters/concerns. c. Periodically conducting surveys/inspections of each Center's food services for compliance with NASA requirements and applicable local, State, and Federal regulations. d. Investigating Agency-level complaints associated with food safety concerns. e. Coordinating with outside agencies, as applicable, on food safety and related environmental health matters. f. Notifying, by the most expeditious means, the Agency Director of Occupational Health of food-related incidents as they are reported by the Centers. 4.10.3.2 Center Directors, including Headquarters and Component Facility Directors shall be responsible for: a. Ensuring compliance with the provisions in this section. b. Ensuring all Center Food Safety Program elements are implemented and maintained. 4.10.3.3 Centers shall be responsible for: a. Providing for design/procedure reviews, technical assistance, and consultation with all Center organizations on matters concerning food sanitation. b. Performing, at least quarterly, on-site, risk-based, food safety audits of food establishments; identifying and assessing the hazards and associated risks; determining and implementing critical controls and procedures necessary to reduce risk of foodborne illness; and providing a program of active managerial control. c. Maintaining accurate and complete survey and inspection data records for the last three months or last three inspections, whichever time span is greater. d. Providing consultation in the preparation of State and/or local permit applications for food service activities and maintaining copies of those documents and permits, with associated records, to ensure compliance monitoring and reporting is carried out in a timely manner. e. Identifying and providing support concerning certification and training requirements for food managers and food service workers in order to promote a comprehensive awareness and active managerial control of risk factors most commonly associated with foodborne illnesses. f. Notifying, by the most expeditious means, the SEHO of major food-related incidents as they are reported. g. Correcting all inspection violations in a reasonable length of time. h. Notifying their local and State health departments, the Centers for Disease Control and Prevention (CDC), and/or the Department of Homeland Security, as applicable, of foodborne disease outbreaks. 4.10.4.0 Process Description 4.10.4.1 Definitions a. Active Managerial Control - the purposeful incorporation of specific actions or procedures by management into the operation of their establishment to attain control over foodborne illness risk factors. b. Design Review - A formal documented and systematic examination of a design to evaluate food service facilities and/or operations, including: food service facilities, water system construction or modifications, and sanitary facilities for buildings. c. Food Establishment - Any operation, including childcare and NASA Exchange-operated facilities, that stores, prepares, packages, vends, or otherwise provides food for human consumption at NASA facilities or on NASA property. d. Food Inspectors - Persons who have displayed knowledge of the primary area of food inspection and regulation and have received certification and/or standardization from an agency that regulates the food industry, or has been credentialed by a state or the National Environmental Health Association. e. Food Manager Certification - A written certification test that requires food managers to demonstrate a basic knowledge of food protection practices. f. Hazard - A biological, physical, or chemical property that may cause a food to be unsafe for human consumption. g. Hazard Analysis Critical Control Point (HACCP) Methodology - A prevention-based food safety management system that identifies and monitors specific food safety hazards that can adversely affect the safety of food products. i. Major Food Safety Incident - Any of the following or related events occurring at an establishment on NASA property or regularly serving NASA personnel: a known poisoning resulting in hospitalization; two or more suspected poisonings; any known or suspected incident of food contamination resulting or potentially resulting in exposure to personnel; or any similar or related incidents. j. Potentially Hazardous Food - A food that is natural or synthetic and that requires temperature control because it is capable of supporting: ? the rapid and progressive growth of infectious or toxigenic microorganisms, ? the growth and toxin production of Clostridium botulinum , or ? in raw shell eggs, the growth of Salmonella Enteritidis. Includes foods of animal origin that are raw or heat-treated; foods of plant origin that are heat-treated or consist of raw seed sprouts, cut melons, and garlic in oil mixtures that are not acidified or otherwise modified at a processing plant in a way that results in mixtures that do not support growth of pathogenic microorganisms as previously described. k. Risk-Based Inspection - An assessment of the degree of active managerial control that an operator has over the foodborne illness risk factors in the establishment; and the focusing of inspections on the control of foodborne illness risk factors, which embody a preventive rather than reactive approach to food safety. l. Risk Factor - One of the broad categories of contributing factors to foodborne illness outbreaks, as identified in the CDC Surveillance Report for 1993-1997, that directly relates to foodborne safety concerns within retail and food service establishments. The factors are: Food from Unsafe Sources , Inadequate Cooking Temperatures , Improper Holding Temperatures , Contaminated Equipment , and Poor Personal Hygiene. m. Temporary Event - A food establishment that operates in conjunction with a single event or celebration. n. Vector - An organism that is capable of transmitting a pathogen from one organism to another. o. Vermin - Any of various small animals or insects that are destructive or pose a health hazard to humans, plants, or animals in the environment. 4.10.4.2 Provisions 4.10.4.2.1 General a. All food served or vended for human consumption shall be stored, handled, prepared, and dispensed in a manner which ensures food safety. b. Vermin shall be controlled to prevent the creation of a health hazard to humans. 4.10.4.2.2 Constraints/Controls Constraints/controls imposed upon substances/operations subject to the provisions of this section shall be no less than those required by applicable regulatory authorities and shall include any additional special constraints deemed necessary by the NASA SEHO as a result of unique or operational characteristics. 4.10.4.2.3 Records All records generated by following these procedural requirements, including but not limited to those required by local, State, or Federal statute or regulation, and the most recent edition of the FDA Food Code, shall be maintained in accordance with NPR 1441.1, NASA Records Retention Schedules. All records shall be available for review by the NASA SEHO or designee and Federal, State, and/or loca
, l food safety inspectors. Examples o
, f records include applicable training records, inspection records, temperature logs, food receiving logs, maintenance logs, and dishwasher logs. 4.10.4.2.4 Waivers Waivers from some or all NASA Food Safety Program requirements described in this section shall be determined on a case-by-case basis by the NASA SEHO. Requests for waivers shall be submitted to the NASA SEHO in writing for approval by the Chief Health and Medical Officer (CHMO) prior to their initiation. Authorizations may be withdrawn at any time for violations of the granted waiver or other regulatory non-compliance. Centers shall provide copies of requests for waivers to the Office of Safety and Mission Assurance if they are deemed to affect the NASA workforce, and the appropriate union, as applicable. 4.10.4.2.5 Non-Compliances Where Agency surveys or inspections indicate non-compliance with NASA-approved procedures and controls, the responsible organization for that activity shall correct all discrepancies and notify the NASA SEHO and the Center Occupational Health Office when remediation has been completed. 4.10.4.2.6 Accidents, Incidents, and Emergencies The NASA SEHO and the Center Occupational Health Office shall be immediately advised of major accidents, incidents, or emergencies involving food safety. 4.10.5.0 Safety 4.10.5.1 General This section outlines the basic methods to prevent outbreaks of foodborne illness by assuring all food served or vended at NASA Centers and Component Facilities shall be clean and free of pathogenic organisms, contamination, and organic or inorganic toxins (including those of bacterial origin). This applies to transporting, storing, preparing, serving, and vending of food provided/available at NASA Centers and Component Facilities. This applies equally to all appropriated funded, non-appropriated funded, organizational, contractor, and/or private association food activities held on NASA Centers and Component Facilities. Centers and Component Facilities shall comply with HACCP principles and the United States Department of Health and Human Services, FDA Food Code. 4.10.5.2 Organizational Functions 4.10.5.2.1 Centers shall: a. Ensure that the requirements and provisions of this section are included in all food and beverage services contracts, subcontracts, and other applicable contracts. b. Conduct a continuing program of inspection and surveillance of all food establishments by individuals who are qualified by certification or standardization to conduct inspection and surveillance to ensure that all food service establishments shall meet or exceed the minimum acceptable requirements established by NASA directives, as well as applicable Federal, State, and local regulations for the safe handling of food. Such inspections shall be performed under the requirements established by this section, the FDA Food Code, and other applicable Federal, State, and local regulations. c. Remove from service or sale all food items suspected to be contaminated, unwholesome, or otherwise deemed unfit for consumption. d. Ensure design reviews are conducted for new or redesigned food establishments, as well as for facilities that intend to make significant changes to the existing menu or theme. Facility plans shall consider menu plans and include equipment specifications and food establishment facility layouts. e. Review prepared food establishment design review packages that include proposed menu, equipment specifications, and equipment layout for any food facility to be newly constructed, remodeled, or reopened. f. Review plans for temporary events and provide recommendations concerning food safety provisions. g. Ensure that Center policy requires prompt notification of a responsible individual in the event of an emergency such as a fire, flood, power outage, or similar event which might result in the contamination of food or that might prevent potentially hazardous food from being held at safe temperatures. h. Ensure that training is provided to maintain certification requirements for all food service employees. i. Maintain the most recent copy of the inspection form and have it available for review by inspection personnel and food installation customers. j. Ensure all food handlers report to their designated medical clinic or designated medical care provider when any symptoms of infections and/or communicable disease are present. k. Ensure that all food handlers returning to work after an illness-related absence associated with any of the conditions below are cleared by an appropriate medical care provider: i. A diagnosed illness of Norovirus, typhoid fever (Salmonella typhi), shigellosis (Shigella spp.), E. Coli O157:H7 infection (or other EHEC/STEC (enterohemorrhagic or Shiga toxin-producing E. Coli)), or hepatitis A virus (hepatitis A)). ii. Symptoms of gastrointestinal illness such as diarrhea, fever, vomiting, jaundice, or sore throat with fever. iii. A lesion, boil, or wound containing pus that is open or draining and is on the hands, wrists, or exposed portions of arms. iv. Illness from consuming food that was implicated in or caused an outbreak. l. Ensure that organizations operating vending machines that dispense food or beverages provide the Center Occupational Health Office with a list of on-site vending machines and their locations, where potentially hazardous food items are dispensed. 4.10.5.2.2 Centers shall have a written food safety policy that requires them to ensure that an HACCP or equivalent management system includes and implements a process of self-inspection and continuous improvement. The management system shall provide for active managerial control and the purposeful incorporation of specific actions or procedures into the operation of food service establishments to attain control over foodborne illness risk factors. Unique conditions within each facility shall be considered during the development of food safety plans. A generic plan is not acceptable. The food safety plan shall identify potential hazards of significance and include preventive measures to ensure and improve food safety. Critical Control Points shall be effectively controlled. 4.10.5.3 Equipment/Facility Design Features 4.10.5.3.1 New and redesigned facilities shall be reviewed by the Center Occupational Health Office. The design of a food establishment shall meet the principles outlined in State and local codes and the FDA Food Code, unless they cannot be technically accomplished. All design deviations shall be approved by the Center's Occupational Health representative prior to implementation. 4.10.5.3.2 Changes that may affect the Center's Food Safety Program requirements shall be coordinated with appropriate Center personnel and approved in advance by the Center's Occupational Health representative. 4.10.6.0 Bottled Water for Water Dispensers 4.10.6.1 General This subsection contains requirements to ensure all Centers' bottled water dispensers are in compliance with Federal, State, and local regulations. 4.10.6.2 Organizational Functions The Center shall maintain a continuing surveillance of all bottled water dispensers for compliance with NASA, Federal, State, and local standards. 4.10.6.3 Requirements Only bottled water approved by the Center shall be placed in bottled water dispensers. Under no circumstances shall empty bottles be refilled by anyone other than the processor. All organizations procuring bottled water shall ensure that: a. No bottled water dispensers are allowed or bottles of water stored, in areas where general hazards or contamination of any kind pose a threat to users under normal operations. b. Contractors and subcontractors furnishing bottled water shall provide routine chemical and microbiological laboratory analysis reports for bottled water delivered to the Center. c. Bottled water dispensers are maintained in a sanitary condition. d. All dispensers have equipment numbers. e. There is prompt recall of the suspect bottled water and/or other appropriate action when notified of contamination detection. 4.10.7.0 References b. 34 CFR 395.32, Collection and distribution of vending machine income from vending machines on Federal property. c. NPD 1820.1B, NASA Environmental Health Program. d. NPD 9050.6I, NASA Exchange and Morale Support Activities. e. NPR 1441.1, NASA Records Retention Schedules. f. Diagnosis and Management of Foodborne Illnesses, A Primer for Physicians and Other Health Care Professionals, and Introduction and Clinical Considerations. Web site: http://www.ama-assn.org/ama1/pub/upload/mm/36/2004_food_introclin.pdf . g. HACCP-Based Standard Operating Procedures (SOPs). Web site: http://sop.nfsmi.org/HACCPBasedSOPs.php . h. Hand Hygiene in Retail & Food Service Establishments. Web site: http://www.cfsan.fda.gov/~comm/handhyg.html . i. Managing Food Safety: A Manual for the Voluntary Use of HACCP Principles for Operators of Food Service and Retail Establishments. Web site: http://www.cfsan.fda.gov/~dms/hret2toc.html . j. Managing Food Safety: A Regulator's Manual for Applying HACCP Principles to Risk-based Retail and Food Service Inspections and Evaluating Voluntary Food Safety Management Systems. Web site: http://www.cfsan.fda.gov/~dms/hret3toc.html . k. The Bad Bug Book. Web site: http://www.cfsan.fda.gov/~mow/intro.html . l. U.S. Department of Health and Human Services, Public Health Service, Food and Drug Administration, FDA Food Code, with changes (most recent edition). Web site: http://www.cfsan.fda.gov/~dms/foodcode.html . m. U.S. Food and Drug Administration, Center for Food Safety and Applied Nutrition, Web site: http://www.cfsan.fda.gov/~dms/hret2toc.html . 5.1.1 Introduction The NASA Employee Assistance Program (EAP) is a
multifaceted approach for assisting employees and their immediate families to
address work and family life issues that might affect the employee's health
and well-being, the safety of the employee and coworkers, or job performance and
productivity. The EAP provides
confidential services and ensures privacy and confidentiality with a few
specific exceptions. The service is
free for all NASA civil service employees. 5.1.2 Responsibilities a. All NASA
Centers are responsible for providing adequate professional staffing to ensure
an effective EAP program. b. The NASA
EAP Managercoordinates Center activities, implements
Agency EAP plans and programs, works to assure confidentiality, and provides
assistance to EAP professionals regarding implementation of Center programs such
as smoking cessation and stress reduction. 5.1.3 Process Description a. Short-term
counseling involves from one to several sessions, over a discrete period of
time, as determined by the EAP professional.
EAP counseling service does not include clinical evaluation or diagnosis
but constitutes at least the following: (1) Counsels employees by voluntary self-referral or
by supervisory referral to the EAP. (2) Informs employees or other eligible participants
of client confidentiality rights and of length and type of services
provided by the EAP. (3)
Provides problem assessment, use of constructive confrontation and
short-term intervention and assists with providing information for referrals
directed to community-based resources. (4)
Refers clients for other assistance and treatment, advising on cost of
any such outside treatment which must be borne by the client. (5)
Follows up on each case to ensure that continuity of care is provided or
to identify reasons the client did not complete care. (6)
Collects overall metrics (without references that can be traced to an
individual client) on numbers and types of cases, mandatory and nonmandatory
referrals, and general demographic data. (7) Establishes
employee feedback and quality control measures to document the degree of
effectiveness of programs while assuring confidentiality. b. The EAP
professional is involved in developing and participating in work/life programs. This includes programs such as Critical Incident Response or
Prevention/Threat Assessment teams, American with Disabilities Act, and
Drug-Free Workplace Program (DFWP) groups.
At each Center, the EAP professional is the designated point of contact
for the above programs who is responsible for the following: (1)
Works with Center employees and management groups to provide for early intervention
and awareness of the programs offered.
(2) Offers
training and consultation to employee groups. (3) Establishes
employee feedback and quality control measures to document the degree of
effectiveness of programs while assuring confidentiality. 5.1.4 Flow Diagram The flow diagram for this process is shown in Figure at the end of this section. 5.2.1 Introduction Workplace violence has become an important health and safety
concern in today's workplace. Acts
of violence are costly in terms of lost workdays, lost productivity, and
increased healthcare expenditures. Violence
is defined as any act against an individual that creates a hostile environment
and/or negatively affects an individual either physically or psychologically.
NASA's top priority is the health and safety of the visiting public,
the astronauts, and employees. Tolerance
will not be shown, therefore, for acts of violence, harassment, threats,
intimidation, and other disruptive behavior. 5.2.2 Responsibilities a. NASA Center
Human Resources personnel ensure that employees are informed of Center's
policy, ensure a mechanism to report concerns or incidents, and coordinate
necessary actions when an incident has occurred. b. EAP and medical services personnel ensure training
is available on violence and stress management. c. Center
Medical Directors assist EAP personnel with training of Center personnel to
reduce workplace violence, treat medical emergencies associated with an incident
of workplace violence, and evaluate or refer for evaluation suspected cases
involving a psychiatric, alcohol or drug-related problem d. Security personnel respond to
all potential incidents and provide protection with as little disruption as
possible. 5.2.3 Process Description: a. Develop a Center policy concerning workplace
violence in all forms. b. Ensure training on Center policy, stress
management, and workplace violence. c. Identify problems early and resolve them to
prevent workplace violence. d. Identify in the policy the mechanism, person, and
telephone number to contact if there is an emergency situation and if there
is a nonemergency developing problem or concern. e.
Ensure that the security personnel are well-trained to handle developing
situations with as little disruption and escalation of the situation as
possible, while iensuring Center and personnel safety. f.
Evaluate psychiatric, alcohol, or drug-related behavior. g. Address with employees the stress, grief, and
security concerns if a workplace violence situation develops. 5.2.4 References: a.
The CDC Web site at www.cdc.gov/niosh/violdev.html b.
The OSHA Web site at www.osha.gov/oshinfo/priorities/violence.html 5.2.5 Flow Diagram The flow diagram for this process is shown in Figure
5.2 at the end of this section. 5.3.1 Introduction a. Domestic
violence is chronic abuse by one current or former intimate partner against the
other or their dependents. It is characterized by a pattern of
coercive control and increasing entrapment.
Occurrence and severity of domestic violence may increase with stress,
financial difficulties, and job insecurity. b. Awareness
of the potential for domestic violence and steps to be taken to prevent it are
important to ensure the safety of the affected employees.
It will help to assure a safe and productive working environment.
Employees must be provided with education and resources to increase their
awareness that may help prevent or stop domestic violence.
It is important for supervisors and coworkers to understand the nature
and dynamics of domestic violence to help provide proper intervention. 5.3.2 Responsibilities a. The employee's supervisor is the key individual for
identifying problems through observing unusual performance, physical evidence of
repeated trauma, and emotional problems that need to be addressed. b. Center EAP
personnel provide consultation to management on employee problems, allow for
easy confidential access to services, and provide information on community and
Center contacts concerning domestic violence. c. The Center Medical Directors ensure that health care workers
take domestic violence seriously, screen employees for domestic abuse, provide
information on domestic violence, make referrals to available resources, and
take appropriate action concerning possible domestic violence. d. The Human Resources Director works with EAP and
medical personnel to assist with job related support needs. e. NASA
Center Directors ensure that plans are in effect to assure employee safety at
their Centers. In addition, they
ensure professionals are available at the Center to confidentially address
employee domestic violence concerns. 5.3.3 Process Description a.
A Center policy is prepared for a multidisciplinary approach to identify,
address, and ensure Center safety for the domestic abuse victim.
The following areas need to be addressed: (1)
Confidentiality must be emphasized and maintained. (2)
Security of the employee and coworkers must be emphasized, especially if
the abusive partner works at or has access to the Center. (3) Access to
resources at the Center is essential, as the abused partner may not be free to
access services after work hours. (4) Center EAP,
supervisory, medical, and Human Resources (HR) personnel must understand their
respective functions in dealing with domestic violence). b. ALL reports of abuse must be taken seriously and
the individual referred appropriately for assistance. c. Information on personnel to contact, telephone
numbers to call when someone needs assistance, and a list of community
resources should be available onsite, as well as in confidentially
accessible locations such as female bathrooms d. Training and lectures should be made available to
employees that address-- (1) Center violence policy. (2) Resources available. (3) Emotional support and self-esteem or empowerment. (4) Dynamics of abuse and barriers to ending
domestic abuse. (5) Financial, legal, and advocacy needs. (6) Security questions. e. Medical staff provide instructions on the physical
and behavioral signs of abuse. f. EAP personnel should be prepared to perform the
following: (1) Assess danger risk to employee from abusive
partner. (2) Provide
contacts and telephone numbers for dealing with the abusive situation in the
community, and encourage employees to memorize emergency numbers. (3) Arrange for
onsite contact if community resources are not easily available. (4)
Develop a "safety plan" with the affected employee to include-- (a) Preparation to leave the abusive partner,
including financial preparation, rehearsal, and necessary documents to
take. (b) Protection during violent incidents. (c) Safety in the home. (d) Safety arrangements at work or public places. g. Human
resources, legal, and security personnel should be prepared to assist, if
necessary, when the abusive partner has access on the Center to the employee
affected by domestic violence. 5.3.4 Flow Diagram The flow diagram for this process is shown in Figure
5.3 at the end of this section. 6.1.1 Introduction a. The
prevention and management of occupationally related injuries and illness is a
key function in ensuring the health and safety of the NASA workforce.
Timely reporting and early medical intervention following an injury or
illness helps to facilitate the recovery process and reduce the potential for
disability. Case management for cases involving lost time
injuries/illnesses facilitates a safe and timely return to work utilizing
modified duty and/or work accommodations. These
efforts are critical to reducing days away from work and reducing the costs
associated with injuries and illnesses. b. Case
management is a collaborative process that assesses, plans, implements,
coordinates, monitors, and evaluates options and services required to meet
individual health needs, using communication and available resources to promote
quality and cost-effective outcomes. 6.1.2 Responsibilities a. The Center Medical Director is responsible for the medical aspects of
civil service workers' compensation cases.
This includes initiating and providing medical evidence to NASA
compensation personnel for claims that do not appear to be work-related and
should be refuted. The respective
Center Human Resources Office (HRO) and Compensation Claims Office (CCO) will
assist as required. b. The NASA OHP establishes the responsibilities and
procedures for the workers' compensation program. The OHP is responsible
for the evaluation of workers' compensation data trends and monitoring
costs Agencywide. 6.1.3 Process Description a. Center OHP
personnel ensure that treatment options are available for any employee with an
occupationally related injury or illness during and after normal business
hours. (1) The employee is responsible for reporting the
injury or illness and seeking treatment. (2) The health care provider obtains history,
assesses injury or illness, and provides treatment. (3) Ongoing care and followup are provided until
issue is resolved or Maximum Medical Improvement (MMI) has been reached. (4) A determination of the ability to return to work
and the need for job modifications or accommodations is made. b.
Case managers are essential when a case involves lost time, needs medical
limitations, or the case has been managed by a health care provider outside of
the OHP clinic at the Center. Duties
of case managers include the following: (1) Supporting
employee throughout the treatment and return-to-work process. (2) Monitoring
appropriateness and effectiveness of care being provided. (3) Assessing
employee compliance with treatment recommendations. (4) Facilitating
early and safe return to work within medical limitations. c. Assistance from the Offices of Human Resources and
Compensation Claims will be requested as needed. d. Human
Resources Office personnel work with the Center Medical Director as an employee
is cleared for return to work, modifying duties in a job description as needed
to expedite a productive return and transition to full duty. e. CCO's are
responsible for working with employees to assist in filing claims and working
with the Center Medical Director as needed for potential cases of controversy.
The latter may require advice from the Office of Chief Counsel. f. The NASA OHP is kept apprised of all WCP actions.
6.1.4 Reference a. NPD 1840.1A,
NASA Workers' Compensation Program, dated February 23, 2001. b. NPR 1840.1 Management of Workers'Compensation
Injuries and llnesses, August 23, 2001. 6.1.5 Flow Diagram The flow diagram for this process is shown in Figure
6.1 at the end of this section. 6.2.1 Introduction The Office of Workers' Compensation Programs (OWCP)
determines the information and timeliness requirements for processing civil
service workers' compensation claims and reports. Prompt processing of WC claims through a case management
system assures the most expeditious authorization of medical care for the
injured and facilitates the return of the injured employee to productive
employment. Management of a case is
a process used to track medical treatment and return to work of the injured by
the Center WC Officer or in combination with Center medical, human resources and
safety personnel, as appropriate. 6.2.2 Responsibilities a. Employees are responsible for notifying the Agency
of injuries. b. The WC Officer for each Center issues or authorizes
issuance of OWCP forms and tracks status of claims and claim forms. c. The supervisors of the injured employees complete
their portion of the OWCP forms within 2 working days and return the form
to WC Officer. d. The OHP monitors Agency OWCP data trends and costs. 6.2.3 Process Description a. Continuation of Pay (COP) The WC Officer reports COP on a quarterly basis.
Reports are completed and submitted no later than January 15, April 15,
July 15, and October 15, for the quarter ending the preceding month. The
report includes the number of COP cases listed by claimant name; hours, and
cost of COP; number of full, partial, and calendar days of COP used for
that quarter; and for payments made in that quarter, and for COP incurred
in a previous quarter. The WC Officer controverts payment of COP if
medical evidence of injury is not provided. b. Reserved (1) Reserved. (2) Reserved. (3) Reserved. (4) Reserved. c. Reporting The WC Officer maintains a record of the date of injury,
dates of issue of CA-1, CA-2, or CA-16 forms, whether the claim was first aid
only, the date (if applicable) when the first- aid injury became a nonfirst-aid
reportable injury, and when the initial claim forms CA-1, CA-2, and CA-7 were
sent to OWCP. Reporting follows the sequence below: (1) Employee notifies Agency of injury. (2) Appropriate OWCP forms issued by Center. (3) Employee completes form, obtains appropriate
medical documentation. (4) Employee's supervisor completes appropriate forms. (5) WC Officer verifies completeness of form, supports or
controverts payment of COP, and challenges nonwork-related claims. (6) WC Officer verifies a case management process to
facilitate appropriate medical treatment and prompt return to work. (7) WC Officer reports COP and pertinent injury data to the
OHP for Occupational Health and to their Center management. 6.2.4 Flow Diagram The flow diagram for this process is shown in Figure
6.2 at the end of this section. This chapter establishes a method for performing and documenting the
results of Agency Occupational Health reviews and delineates requirements
for biennial evaluations of major NASA HQ and field locations that occur
on NASA property. For purposes of the audit element of the review process,
the OCHMO defines a requirement as a mandatory element for a program or
function. Requirements shall include NASA Policy Directives (NPDs), NASA
Procedures and Requirements (NPRs) and other external regulations and
consensus standards applicable to NASA. The review process shall be comprehensive enough to provide Center
Senior Management with a status of the effectiveness of their Center's
programs. By definition this must include the provision of sufficient
resources commensurate with the Center's size, population, and mission to
achieve and maintain desired effectiveness. Regular reviews of medical and occupational health services are required
to assure protection of the health of the NASA workforce. Biennial OCHMO
reviews shall be designed to help the Agency identify and mitigate risk,
provide a consistent, high level of health care to participants across the
Agency and identify best practices and innovative solutions that provide
greater operational effectiveness and efficiency. Occupational health (OH) reviews include a compliance audit component.
They also provide an opportunity for open dialogue with Center personnel
and are used as a forum to advocate for appropriate support for health-
related services. The Review Team provides technical help, guidance on best
practices, support on Agency occupational health initiatives, and
facilitates specialized training for emerging health threats or new
requirements as needed to enhance the competency of health discipline
employees. The OH components assessed shall include medical care provided at each
Center's Occupational Medicine Clinic (including emergency care capability
and coordination with other departments, medical quality assurance, and
health clinic environment of care); preventive health activities; the
employee assistance program; federal workers' compensation; fitness
facilities; industrial hygiene; health physics; sanitation and food safety
programs; and medical aspects of other programs such as emergency
preparedness and childcare facilities. During the alternate years, when a review is not conducted at a Center,
the Center shall perform a self evaluation using criteria provided in the
most recent occupational health review conducted at the Center, or some
other approved equivalent methodology. A record of areas reviewed, names
of individual(s) conducting evaluations, dates and locations shall be
retained on file at the host center. Each Center shall provide a self-
evaluation to the OCHMO in the same month of the alternate year in which
they are normally audited. The Center report shall delineate areas of
substantial improvement and/or degradation of their occupational health
program. Occupational health reviews shall compare Center policies, procedures,
and practices to regulatory and other compliance requirements, agency
policy requirements and consensus standards. A written report shall be
produced from the review findings and provided to the Center for response.
The appropriate Mission Associate Administrator, Institutional Corporate
Management, and Safety and Mission Assurance Directorates shall be
appropriately copied for their reference of findings associated with
health, environmental compliance and/or safety, respectively.
Nonconformance findings shall be tracked to closure as further detailed
below. Occupational health reviews shall begin with scheduling review dates
with the Centers' occupational health Contracting Officer Technical
Representative(s) (COTR) or their appointed representative. The COTR or
their representative shall act as the Centers' primary point of contact for
the review process. The schedule for the program surveys is as follows: KSC - January MAF - February SSC - February GRC - May LaRC - June ARC - August DFRC - August WSTF - September JPL - January JSC - February GSFC - May WFF - May HQ - August MSFC - September A letter shall be sent to the Center Director or Facility Manager, at
least 60 days ahead of the review dates, announcing the upcoming review and
requesting the associated support. This shall be followed with an e-mail
to the COTR containing the review questionnaires and a request for specific
Center documentation. The COTR shall distribute the requests to the Centers'
occupational health representatives. The Centers' occupational health
representatives shall answer the questions. The primary point of contact
shall return the questionnaires to the OCHMO Review Team Leader via e-mail.
The requested Center-specific documentation shall be returned to the OCHMO
Review Team Leader, who will distribute it to the Review Team for their
assessment prior to the Center review. The review shall begin with an in-briefing of the
principals involved in the review process. The Center shall provide
multi-disciplinary coverage for the entire review period. The in-briefing
shall provide a forum for an exchange of information and details regarding
the review. It shall also provide the Centers with current OHMS
information that may affect them and provide feedback on previous review
process improvements. The in-briefing shall be an opportunity for the
OHMS Review Team to offer expert information and advocacy while on site.
The Centers shall present their top occupational health concerns and a
status of any open or unresolved nonconformance findings from previous
occupational health reviews at the in-briefing. After the in-briefing, each reviewer shall meet with
their subject matter counterpart to clarify questionnaire answers, discuss
Center occupational health programs and processes and plan area visits.
Information shall be collected and verified through interviews, tours of
work areas, observation of activities and the surrounding work environment
and conditions, documentation of reviews, and record inspections. The out-briefing shall be presented to the Center
Director or the Deputy as a verbal executive summary. It shall focus on
the strengths and weaknesses found during the review. A written report
shall be prepared within 45 days of out-briefing. The written report shall be a reiteration of the same
issues expressed in the verbal executive summary, plus a detailed report of
all review findings. Centers shall have 45 days after receipt of the
written report to reply to the OHMS Review Team Leader with a corrective
action plan that addresses remedial actions for the non-conformance
findings associated with the review process. Nonconformance findings shall be closed within 6
months or as negotiated. The Center shall keep the OHMS Review Team Leader
informed of the status of corrective action activities particularly if a
delay is anticipated. Corrective actions shall only be "closed" when the
anomalous condition associated with the non-conformance no longer exists. A re-visit to a Center to review the occupational
health program, or portion thereof, shall be performed when important
program elements are non-conforming or incomplete or if, in the opinion of
the discipline reviewer and team leader, the program or a portion thereof,
is sufficiently weak or degraded as to require a review before the next
biennial review. Follow-up review elements shall concentrate only on the
deficient areas. Working documents, reports, and results shall be
retained on file or in the Agency Health Electronic Database (AHED) for use
and future examination. Findings shall be categorized as follows: Commendation: Best practices that far exceed
requirements or are ingenious time or cost-saving solutions to problems. Recognition: Acknowledgement of significant
improvements or progress toward Center occupational health program goals. Observation: A noteworthy comment such as a notation
of a significant difference compared with other Centers' practices. Such
comments are neither positive nor negative in nature. Opportunity for Improvement: A condition that meets
compliance requirements, but could be improved. Opportunities for
Improvement will be accompanied by "Recommendations" in the written report
but are not required to be addressed in the Center's corrective action
plan. Nonconformance: A divergence from a compliance
requirement (Federal, State, local, NASA Agency, NASA Center, etc.) or
consensus standard (ANSI, HACCP, NIOSH, NFPA, etc.). These findings
require Center response in the corrective action plan. 7.4.1 The Chief Health and Medical Officer shall be
responsible for: a. Ensuring that planned program evaluations of NASA
facilities are conducted to determine the value and adequacy of Center
occupational health programs and that program resources are appropriately
provided b. Reviewing Flight Medicine aspects of NASA's
occupational health processes c. Determining the need for more frequent specialized
surveys on aspects of, or the entire, occupational health program. 7.4.2 The Director of Occupational Health shall be
responsible for the following: a. Assuring overall occupational health review
process efficacy b. Appointing the team lead for Center reviews c. Reviewing and releasing occupational health review
reports d. Approving or disapproving lessons learned
suggestions prior to their implementation 7.4.3 The OHMS Review Team Leader shall be the Agency's primary
representative for the review and shall be responsible for the following: a. The overall effective implementation of the
Agency's occupational health review b. Initiating contact with each Center prior to
review c. Providing Review Team members with
discipline-specific point of contact information for each Center, including
names, e-mail addresses, and phone numbers d. Coordinating and exchanging information with each
Center primary point of contact e. Establishing each Center's review schedule and
associated meetings f. Representing the OHMS at each Center's review
in-briefing meeting g. Overseeing all aspects of the review on site h. Coordinating real-time issues and problems, as
they arise, during the review process i. Consulting with the Agency's Director of
Occupational Health, as needed during the review, regarding nonconformance
findings j. Representing the OHMS at each Center's out
briefing meetings k. Coordinating and preparing review reports l. Conducting "lessons-learned" meetings with Review
Team Members as soon as feasible after each occupational health review m. Compiling strength and weakness summaries for
inclusion into each Center's written report summary n. Ensuring that the written report is consistent
with the verbal out briefing, and is released within 45 days. o. Tracking Centers' nonconformance findings to
closure p. Continually improving the occupational health
review process based on lessons-learned 7.4.4 Center Directors shall be responsible for the
following: a. Appointing a Center point of contact, with
sufficient authority for reviews, to coordinate Center on-site reviews with
the OHMS, and to provide ready access to facilities, and other logistical
support b. Supporting the review effort with adequate
resources and personnel c. Attending the out-briefing or designating the
Deputy Center Director to attend if he/she is unavailable d. Assuring the corrective action plan addresses all
nonconformance findings e. Providing the corrective action plan to the Agency f. Providing adequate resources to resolve corrective
actions g. Ensuring implementation of the requests for action
designated in the review h. Assuring notification of the OHMS for Center
reviews, audits and visits from outside regulatory bodies, such as the
Occupational Safety and Health Administration
(OSHA), the Nuclear Regulatory Commission (NRC), etc. 7.4.5 Individual Agency Review Team members shall be
responsible for the following: a. Being the subject matter points of contact for
their Center counterparts b. Reviewing returned Center questionnaires, and
previous audit/assessment information prior to the review c. Coordinating and exchanging information with each
Centers' subject matter points of contact d. Establishing individual review times and
coordinating meetings with Center counterparts e. Performing their discipline-specific review of the
Centers' occupational health program f. Reporting real-time issues and problems to the
Review Team Leader, as they arise, during the review process g. Representing the OHMS at the review out-briefing or
coordinating with other Review Team members who will attend the
out-briefing meetings, as applicable h. Coordinating, preparing and entering data into the
AHED i. Providing the team lead with a summary of strengths
and weaknesses of the Center pertinent to their disciplines for the summary
report j. Attending "lessons-learned" meetings as they are
scheduled k. Reviewing draft occupational health review reports l. Reviewing, and either accepting or rejecting,
nonconformance closure rationale m. Updating the status of nonconformance findings in
the AHED n. Coordinating nonconformance findings with the team
leader in support of chain-of-command reporting 7.4.6 The Center primary point of contact shall be
responsible for the following: a. Coordinating and exchanging information with each
review Team Member counterpart b. Providing a discipline-specific point of contact
list to the Review Team Leader, including names, mail and e-mail addresses,
and phone numbers c. Distributing review questionnaires from the Review
Team Leader to Center personnel d. E-mailing completed questionnaires to the Agency
Review Team Leader on time e. Providing other requested documentation via e-mail,
fax, etc. to the Agency Review Team Leader f. Arranging for badging and escort of the Review Team g. Coordinating requirements for bringing review
equipment onto the Center, including any forms that must be completed and
submitted for equipment use at the Center (e.g., laptop PCs, cameras, PDAs,
etc.) h. Arranging for a private work area and logistic
support for the Agency Review Team i. Arranging in-briefings and out-briefings and
associated meeting rooms j. Supporting the review schedule and associated
meetings k. Providing access to Center internal locations
subject to the scope of the review l. Providing on-site access to additional Center
documentation as needed m. Overseeing the review for the Center n. Coordinating real-time issues and problems, as they
arise, during the review process o. Coordinating nonconformance findings as needed with
Center Management p. Representing the Center at the review out-briefing
meetings q. Overseeing preparation of the corrective action
plan r. Tracking Centers' nonconformance findings to
closure 7.4.7 Center Discipline-specific points of contact
shall be responsible for the following: a. Being the subject matter points of contact for
their Agency Review Team counterparts b. Emailing completed questionnaires to the Center
primary point of contact on time c. Coordinating and exchanging occupational health
discipline information with the appropriate Review Team counterpart d. Being available to support their Agency Review Team
counterpart with the review process e. Supporting the review in-briefing meetings f. Coordinating individual review times and meetings
with Agency Review Team counterparts g. Providing objective evidence of implementation of
requirements (e.g., documentation, records, licenses, etc.). h. Escorting Agency Review Team personnel i. Reporting real-time issues and problems to the
Center primary point of contact, as they arise, during the review process j. Coordinating with the Agency Review Team on
specific discipline findings prior to the out briefing k. Representing the Center at the review out-briefing
meetings as applicable 7.4. 8 The OHMS Administrative Assistant to the
Director shall be responsible for: a. Maintaining an overall schedule of each review to
assure that critical milestones are met, such as review announcement
letters, pre-review meetings, review letters, six-month status reports,
lessons learned meetings and responses to corrective action plans b. Assuring review announcement letters are initiated
in sufficient time to allow for OHMS and Center actions on questionnaires
and document requests c. Scheduling in-house pre-review meetings d. Preparing the final report from AHED data e. Preparing the executive summary and detailed report
for distribution to the Center within 45 days of completing the review f. Scheduling lessons learned meetings after reviews g. Providing a lessons learned report a. 29 CFR Part 1960 "Basic Program Elements
for Federal Employee Occupational Safety and Health Programs and Related
Matters." b. 29 U.S.C. 668, Section 19 of the
Occupational Safety and Health Act of 1970, as amended. c. NPD 1210, NASA Surveys, Audits, and Reviews
Policy d. NPD 1800.2B, NASA Occupational Health
Program AAMRO
American Association of Medical Review Officers
ACGIH
American Conference of Governmental Industrial Hygienists
ACIP
Advisory Committee on Immunization Practices
ACLS
Advanced Cardiac Life Support
ACOEM
American College of Occupational and Environmental Medicine
ACS
American College of Surgeons
ACSM
American College of Sports Medicine
AED
Automatic External Defibrillator
AHA
American Heart Association
AME
Aviation Medical Examiner
ANSI
American National Standards Institute
B2-M
Beta-2 Microglobin
BAT
Breath Alcohol Technician/Testing
BBP
Bloodborne pathogens
BLL
Blood lead level
BLS
Basic Life Support
C
Centigrade
CBC
Complete blood count
CCO
Compensation Claims Officer
CdB
Cadmium in blood
CDC
Centers for Disease Control
CdU
Cadmium in Urine
CFR
Code of Federal Regulations
COC
Chain of Custody
COP
Continuation of Pay
CPR
Cardiopulmonary Resuscitation
CXR
Chest X-Ray
DASHO
Designated Agency Safety and Health Official
db
Decibel
DEA
Drug Enforcement Administration
DFWP
Drug-free Workplace Program
DOD
Department of Defense
DOT
Department of Transportation
EAP
Employee Assistance Program
ECG
Electrocardiogram/graph
EO
Executive Order
F
Farenheit
FAA
Federal Aviation Administration
FEHP
Federal Employees Health Program
FFD
Fitness For Duty
GXT
Graded Exercise Test
HAZMAT
Hazardous Materials
HBV
Hepatitis B Virus
HEENT
Head, Eyes, Ears, Nose and Throat
HICPAC
Hospital Infection Control Practices Advisory Committee
HIV
Human Immunodeficiency Virus
HR
Human Resources ICC
Infection Control Committee
ICO
Infection Control Officer
ISO
International Organization for Standardization
IV
Intravenous
JCAHO
Joint Commission on Accreditation of Healthcare Organizations
JHA
Job Hazard Analysis
JSC
Johnson Space Center
KSC
Kennedy Space Center
KMWG
Knowledge Management Working Group
MC
Methylene Chloride
MRO
Medical Review Officer
MRP
Medical Removal Protection
MSD
Musculoskeletal Disorders
NASA
National Aeronautics and Space Administration
NBC
Nuclear, Biological and Chemical
NFPA
National Fire Prevention Association
NIOSH
National Institute for Occupational Safety and Health
NODIS
NASA Online Directives Information System
NPD
NASA Policy Directive
NPG
NASA Procedures and Guidelines
OCHMO
Office of the Chief Health and Medical Officer
OEL
Occupational Exposure Limits
OHP
Occupational Health Program
OSHA
Occupational Safety and Health Administration
OTC
Over The Counter
OWCP
Office of Workers' Compensation Programs
PAAMHR
Predicted Age Average Maximal Heart Rate
Pap
Papanicolaou
PAPR
Partial Air Purifying Respirator
PCB
Polychlorinated Biphenyl
PCO
Principal Center Office
PDR
Physicians' Desk Reference
PEL
Permissible Exposure Limit
PFT
Pulmonary Function Test
PPD
Purified Protein Derivative
PPE
Personal Protective Equipment
ppm
parts per million
PSA
Prostate Specific Antigen
rem
roentgen-equivalent-man
RPR
Rapid Plasma Reagin
RTW Return To Work
SAR
Supplied Air Respirator
SCAPE
Self Contained Atmospheric Protective Ensemble
SF
Standard Form
SMAC
Sequential Multiple Analyzer Computer
SOAP
Subjective, Objective Assessment Plan
STEL
Short Term Exposure Limit
TB
Tuberculosis
TLV
Threshold Limit Values
TSH
Thyroid-Stimulating Hormone
TWA
Time-Weighted Average
U/A
Urinalysis
VAERS
Vaccine Adverse Event Reporting System
VPP
Voluntary Protection Program
WC
Workers' Compensation
ZPP
Zinc Protoporphyrins Reserved. Reserved. Reserved. Reserved. Reserved.
3.3 Emergency Services
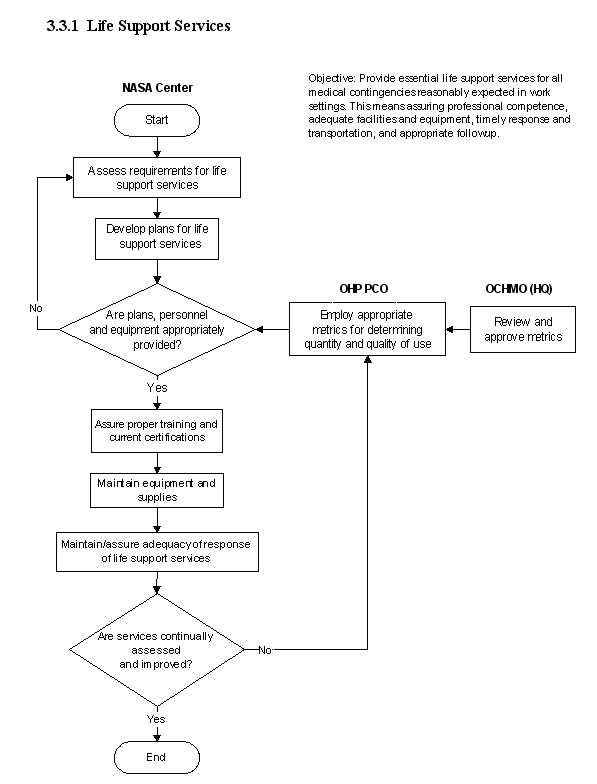
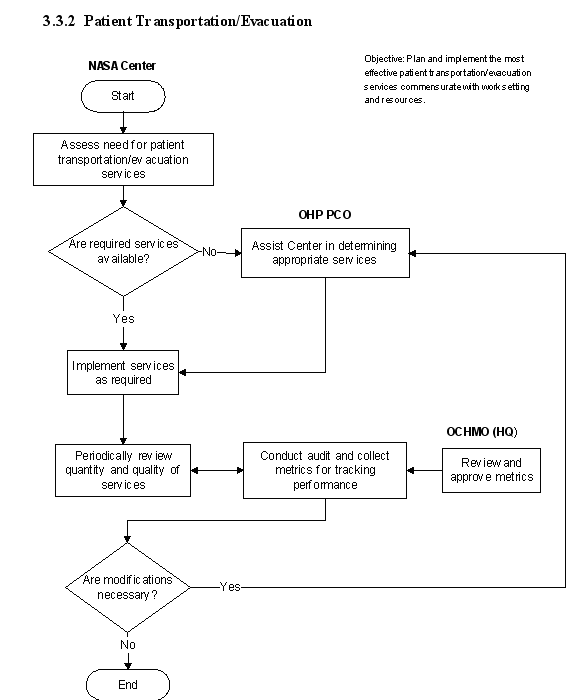

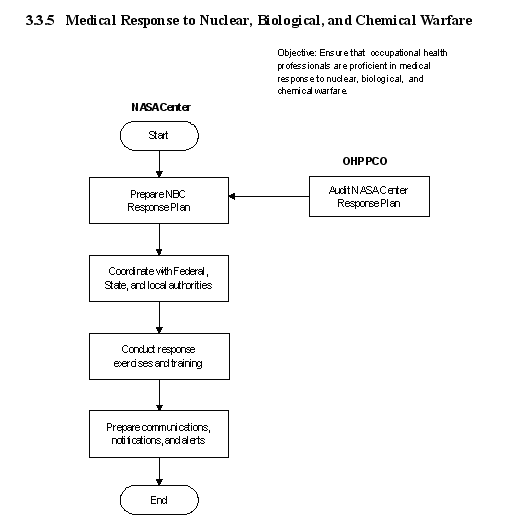
3.4 Infection Control Program
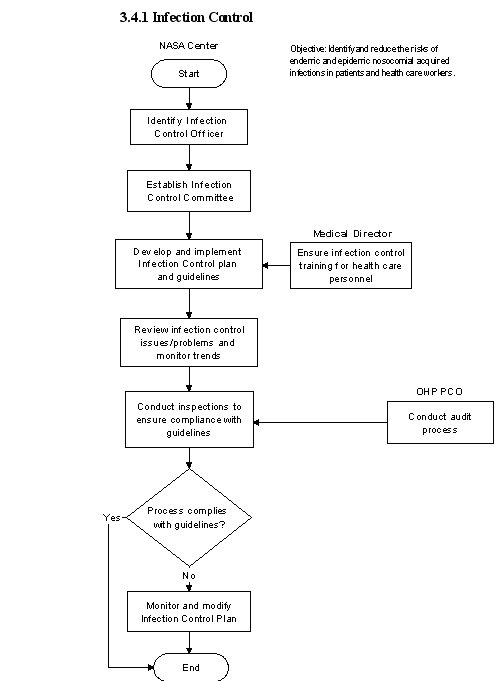
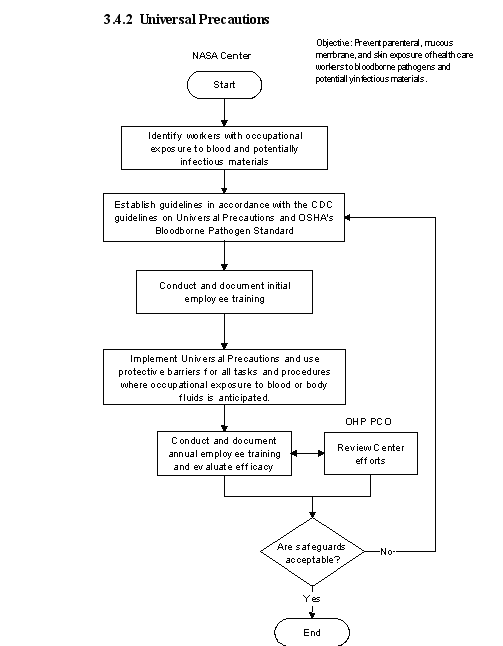

Chapter 4. Environmental Health
4.1 Regulatory Compliance

4.2 Occupational Exposure Assessment and Management
To begin the process by establishing a strategy for exposure assessment.
To collect and organize available information on the workplace; workforce; chemical, physical, and biological agents; existing controls; historical exposure data; biological monitoring data; and any other available source of information.
A complete summary of available essential information on workers, tasks, agents, potential exposures, and potential health effects.
To interpret available information to define exposure groups.
Information and/or data that can be used to enhance the basic characterization and better define exposure groups, their profile, and the risk posed by the exposure profile.
4.3 Occupational Exposure Limits

4.4 Sampling and Analytical Methods and Equipment Calibration
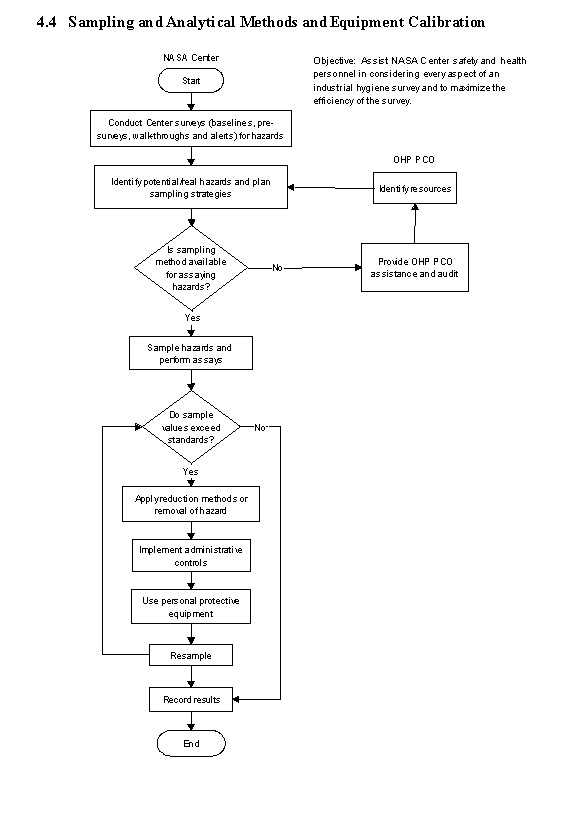
4.5 Ergonomics

4.6 Radiological Health
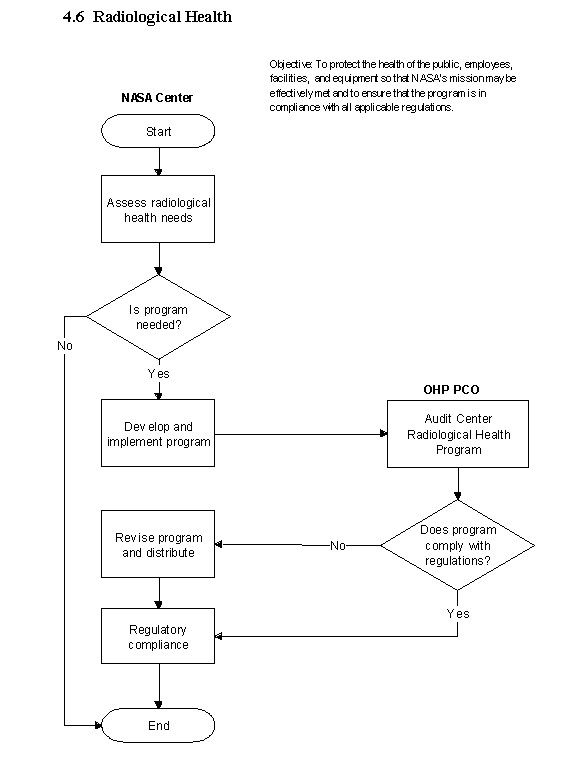
4.7 Environmental Sanitation
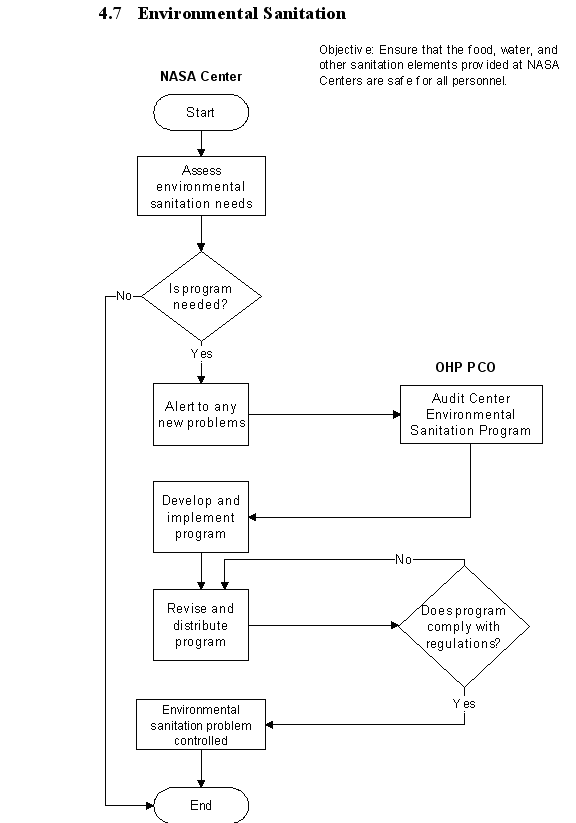
4.8 Balancing Work-Rest Cycles
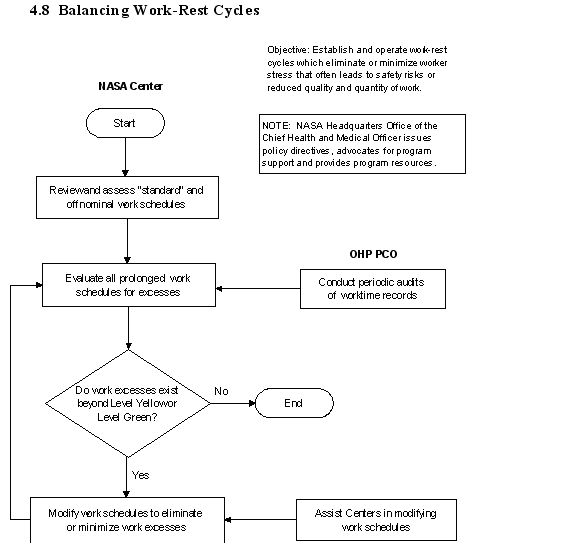
4.9 Hearing Conservation
Exposure level (dBA)
Managing Food Safety: A Manual for the Voluntary Use of HACCP Principles for Operators of Food Service and Retail Establishments (July 2005).
Chapter 5. Employee Assistance Program
5.1 Key Elements of the Employee Assistance Program
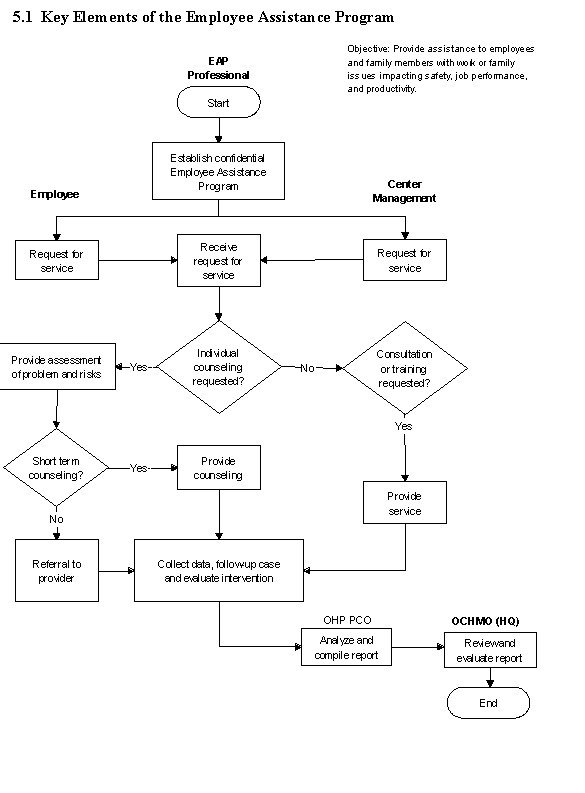
5.2 Workplace Violence
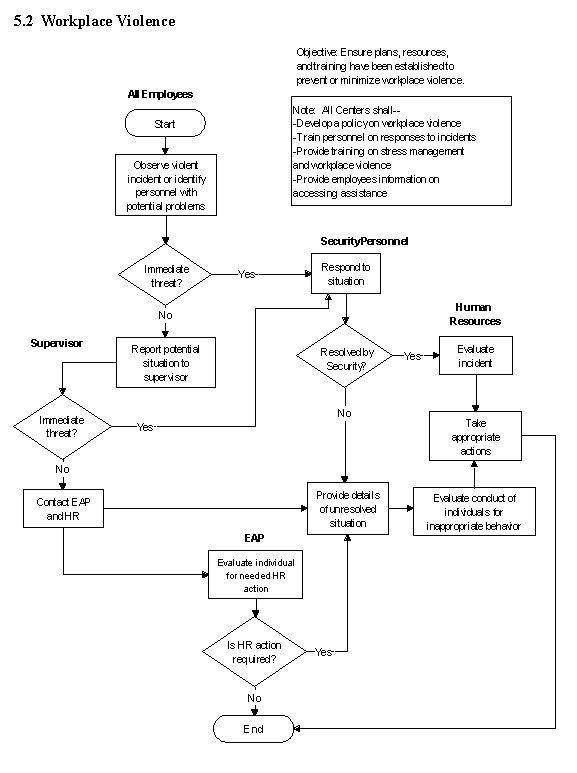
5.3 Domestic Violence
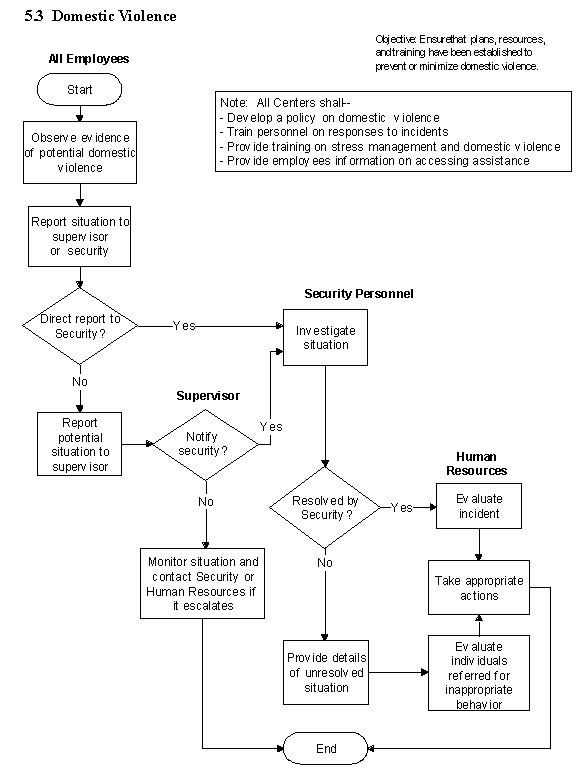
Chapter 6. Workers' Compensation Program
6.1 Management of Workers' Compensation Injuries and Illnesses

6.2 Interagency Reporting
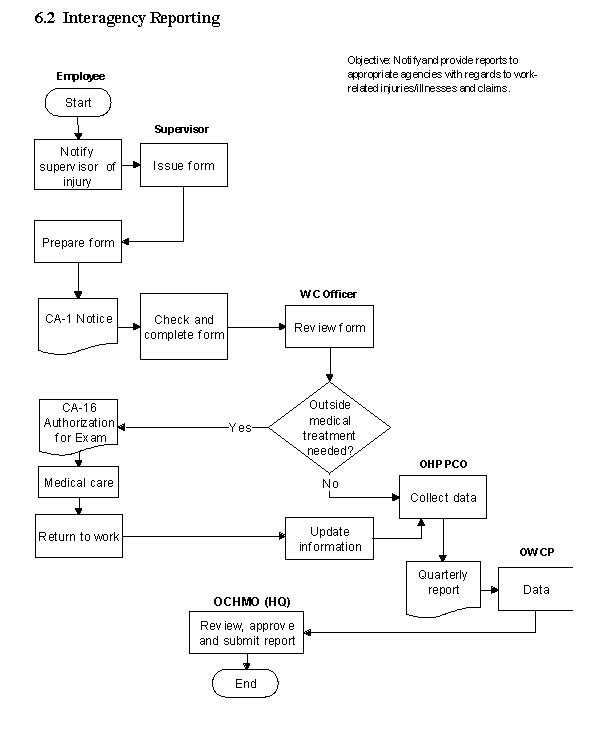
Chapter 7. Occupational Health Review Process
7.1 Introduction
7.2 Purpose
7.3 Process Description
Even Years
Odd Years
7.4 Responsibilities
7.5 References
Appendix A
Acronyms
Appendix G - NASA Physical Examination Matrix
Appendix G is an Excel Work sheet
DISTRIBUTION:
NODIS
This Document is Obsolete and Is No Longer Used.
Check the NODIS Library to access the current version:
http://nodis3.gsfc.nasa.gov