Chapter 3. Occupational Medicine
3.1 Clinical Services
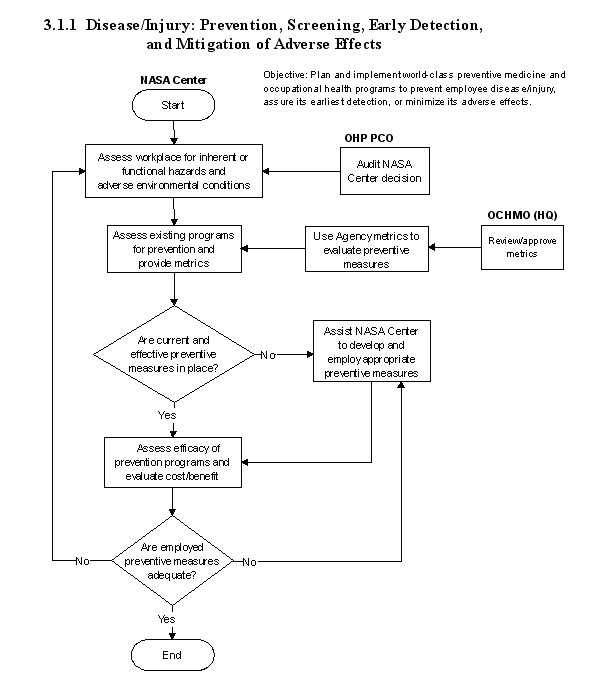
3.1.1 Disease/Injury: Prevention, Screening, and Early Detection, and Mitigation of Adverse Effects
3.1.1.1 Introduction
In the occupational setting, preservation of a healthy workforce is a priority equal to assuring a safe workplace, and in many ways the two are integrally related. NASA's workforce is it greatest strength. Efforts must be directed toward keeping members of the workforce healthy, safe and productive. From a management perspective, the operative concept is prevention. This not only is the least costly in terms of resources expended, but also minimizes the toll in lost work and human suffering.
3.1.1.2 Responsibilities
a. Real and enduring success in maintaining individual as well as corporate health depends upon a continuum of responsibility. Good health results when a culture recognizes the distinctive value of health and is dedicated to its achievement. Prevention begins with the individual.
b. The NASA Center OHP has overall responsibility for maintaining health and safety but the primary responsibility lies at each Center. Prevention really begins as a mind set. OHP personnel at all NASA Centers must think ahead to anticipate hazards, modify processes, and take actions to forestall harmful and injurious conditions and events. This applies to workplace and to worker, and it must be a continuous process.
c. Center OHP personnel must provide preventive services through medical surveillance, health and wellness promotions, immunizations, sanitization of food services, monitoring water supply and control of chemical and physical hazards.
d. The OHP assures implementation of relevant NPD's and regulatory requirements and provides functional management and guidance to health personnel at NASA Centers. It assists NASA Centers in selecting and acquiring resources, contributes to dissemination of information and training of personnel, and helps achieve Aagency-wide uniformity, consistency, and quality.
3.1.1.3 Process Description
a. Successful preventive occupational health services begin with a comprehensive assessment of workplace hazards and adverse environmental conditions. Coincident evaluations of current programs of prevention and of areas requiring preventive emphasis are integrally accomplished. Efficacy of preventive program implementations is determined by metrics appropriately selected to monitor relevant injuries and illnesses as well as achievement of goals and indicators of trends. Critical to such metrics are baseline determinations, and many employees must undergo limited or complete medical evaluations (often termed
"fitness for duty") prior to and during their work in potentially hazardous
conditions. Medical monitoring examinations are performed on employees in an established medical surveillance program. Timeliness of reporting and response are critical to eliminating/minimizing injurious effects of hazards and contraction of illnesses. The OHP encourages establishing and maintaining working relationships between OHP PCO personnel, occupational health management teams, and health professionals at each NASA Center. The work of occupational health preventive services is implemented and enhanced
by onsite visits and audits, planning and training colloquia, management and professional interchange, and instant two-way communication by the latest technologies, including an active NASA OHP Web site.
b. Besides gathering information specific to a given NASA Center, overall Agency statistics are prepared and compared with those of similar industries and settings. Effective preventive health is allied to safety matters and management interests, such as productivity, lost time, workers' compensation, and program costs. The process of benchmarking, always seeking the best and improving status quo, is continuously applied where deemed beneficial. Emphasis on any preventive program effort must match priority and available resources.
3.1.1.4 Flow Diagram
The flow diagram for this process is shown in Figure 3.1.1 at the end of this section.
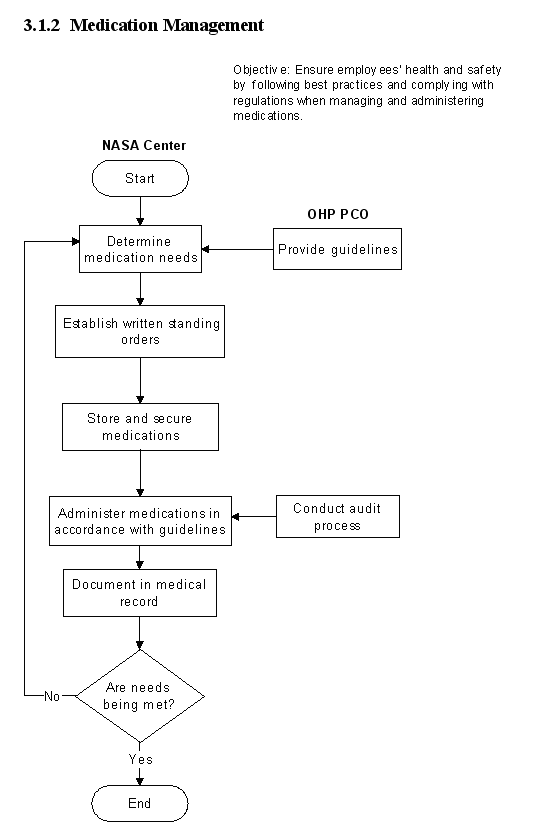
3.1.2 Medication Management
3.1.2.1 Introduction
a. In order to ensure the health and safety of its employees, the Occupational Health clinics must follow best practices and comply with regulations in managing onsite use of medication.
b. Policies and procedures for the control, accountability, and security of all drugs used in Center Occupational Health Clinics must be in place. The clinics must also ensure the competency of their professional staff responsible for administering medications. Additionally, emergency readiness standards and plans are a critical component to the medication management program and must be in place. To facilitate implementation and assessment of this function, the PCO has developed a checklist that Occupational Health personnel may use to assess their program. The checklist is shown in Appendix D.
3.1.2.2 Responsibilities
a. Center Chief Medical Officers/Medical Directors ensure that management of medications in the occupational health facility provides the resources necessary to meet the scope of services provided, and complies with federal, state and local regulations. They also ensure that the program and is administered by competent health care personnel.
b. The OHP is responsible for conducting periodic
audits of the Center's medication management process.
3.1.2.3 Process Description
a. Administration - Regulations
Compliance with Federal and State regulations is essential when managing medications in the workplace. At NASA Occupational Health clinics, the Medical Director assumes responsibility for the management of the medication program. Guidelines for medication
management must comply with the following:
(1) State Nurse Practice Act (defines the scope of nursing practice).
(2) State Medical Practice Act (addresses medications and the delegation from medical to nursing through standing orders).
(3) State Pharmacy Law (defines the terms of prescribing, dispensing and administering of medication and who is legally authorized to do so).
(4) DEA, Controlled Substances Act of 1970 and Executive Order 12564, Drug-Free Federal Workplace, (regulations and laws for managing controlled substances).
(5) Copies of the laws and regulations listed above should be available in the Center Health clinic.
b. Security and Storage
Proper storage and security of medications in NASA
Occupational Health clinics are a key risk-management function that ensures
their integrity and prevents their deterioration.
Guidelines for appropriate storage of medications include the following:
(1) Lock medications in secured areas to avoid unauthorized access.
(2) Store in a secure area not readily accessible to
employees, contractors or visitors.
(3) Store in area of constant supervision or surveillance.
(4) Organize and store for easy retrieval.
(a) Segregate by type, e.g., topical, oral, intravenous, to minimize the risk of medication errors.
(b) Store by therapeutic class rather than
alphabetically to minimize the potential for medication error.
(5) Inspect routinely for expired or deteriorated medication and check that medications are in their designated location.
(6) Store according to the manufacturer's recommendations.
(a) Store in manner that addresses sanitation, temperature, light, moisture, ventilation, and segregation issues to maintain integrity.
(b) Store in a refrigerator, if needed, with adequate storage space.
(c) Store in refrigerator dedicated for medication use only (e.g., should not contain food or biological samples).
(d) Monitor temperature of the refrigerator daily.
(e) Ensure a system to alert of a power loss to the
refrigerator (e.g., such as alarm or clock set on the same circuit).
(f) Monitor storage and security process by periodic random audits.
c. Medication Administration
The medication needs of any clinic must be determined based on the scope of care provided, demographics of the workforce, and nature of operative or potential risks in the workplace. There must be adequate staff qualified, competent, authorized to give medications, and meet State laws.
d. Considerations for the Workplace
There are a number of workplace considerations when
administering medications to workers. Many medications contain substances or compounds, such as alcohol or antihistamines, that may cause drowsiness, impair performance, or potentially cause serious work-related injuries. Medication side effects, such as drowsiness, can impair performance including the operation of heavy equipment, driving a motor vehicle, or interfering with flight operations.
(1) Maintain a current copy of Physicians' Desk Reference and Physicians' Desk Reference for Nonprescription Drugs.
(2) Ensure familiarity of medications' desired and
undesired effects, side and toxic effects, potential interactions, and the
potential allergic reactions.
(3) Assess the potential effects of the medication on employees' ability to perform their jobs safely.
(4) Review current or past health conditions and
prescription or nonprescription drugs including herbal remedies with the
employee.
(5) Consider implications to other medical conditions such as hypertension, glaucoma, or diabetes.
(6) Provide information about the medication, and potential medication interactions.
(7) Promote self-care and consumer awareness about
different products.
e. Standing Orders
Standing orders for all medications available in the NASA Occupational Health clinics provides a standardized approach to providing safe, quality care to employees. Such guidelines and orders establish the standards of care used for peer review and audit purposes.
(1) Establish standing orders for both prescription medicationand Over-The-Counter (OTC) medications available in the clinic.
(2) The drug, dosage, indications, contraindications, and adverse reactions should be included.
(3) The Medical Director and Chief Nurse must write, date, and sign the order.
(4) Standing orders must be reviewed, re-signed, and dated annually.
f. Medication Administration Issues
Prior to prescribing a medication, the healthcare provider must evaluate the patient. The findings of the history and physical examination and the treatment plan must be documented in the employee's medical record. The healthcare provider must discuss the benefits versus the risks of treatment and obtain verbal or written consent from the employee for treatment. The employee's condition must be monitored until the health issue is resolved or employee's care is
transferred to another healthcare provider.
(1) Ensure staff competency through orientation, continuing education, and training.
(2) Provide staff education before new drug(s) added to the formulary.
(3) Ensure familiarity of the medication indications, dosages, side effects, and interactions with other medications.
(a) Review food or herbals, precautions to be taken, and any known allergies the employee may have.
(b) Maintain antidotes on hand in the event of adverse reactions to the medications.
(4) Identify the person, medication, time, dosage, route and technique prior to administering.
(a) Employees should be asked about any known allergies.
(b) Check the expiration date on the medication package.
(5) Provide written patient information sheets.
(6) Accept verbal orders in emergency situations; document as soon as possible.
(7) Verify verbal orders over the telephone after they are written and repeated for the physician, and countersign as soon as possible.
(8) Maintain a sample drugs system that allows for quick retrieval in the event of a recall.
(9) Utilize manufacturer's prefilled syringes with retractable needle.
(10) Utilize eye drop containers on one employee only.
(11) Report medication errors immediately.
(a) Discuss openly and share lessons learned.
(b) Report medication errors to risk management and quality improvement.
(c) Conduct root cause analysis for all medication errors.
(12) Target improvement in medication administration process proactively.
g. Medical Record Documentation
Documentation of the initial evaluation and subsequent
visits must be made in the medical record. The format recommended is the
Subjective, Objective, Assessment and Plan (SOAP). Medication(s) administered must be included in the documentation. Additionally, a list of all medications stocked in clinics must be maintained for prescription, emergency, and controlled substances. Documentation of medications includes the following:
(1) Identify employee allergies or positively note their absence where easily visible on the medical record.
(2) Document on summary sheets significant health
conditions, current medications, and allergies.
(3) Record dosage, frequency, and amount in employee health record.
(4) Record patient instructions and discussion of adverse reactions.
(5) Record lot number for sample medications given.
h. Over-the-counter Medications
The stocking of OTC medications in the Center OH clinics must be based on the needs of the Center population and the scope of occupational health services provided.
(1) Maintain a current copy of the Physicians' Desk
Reference for Nonprescription Drug.
(2) Adhere to standing orders when providing treating.
(3) Ensure adequate packaging and appropriate instructions on the package labeling.
(4) Provide in manufacturer's original unopened container without any type of medical or pharmaceutical intervention.
(5) Utilize unit dose packing to minimize problems
associated with repackaging and cross contamination.
i. Prescription Medications
a. The use of prescription medications at Center Occupational Health clinics is based on need, staffing, and the defined scope of practice. The physician responsible for ordering the
prescription medications must comply with Federal and State laws. Since State laws differ, it is critical they be reviewed for compliance with practices within the clinic setting. For example, State law determines whether prescription medications can be administered per standing orders and whether a physician must be present during the administration of prescription medications. Some States require a dispensing license.
(1) Maintain a current copy of the Physician's Desk Reference.
(2) Maintain an inventory for all prescription medications including sample drugs.
(3) Follow the standing orders when administering medications.
(4) Ensure familiarity with the drugs stocked, including the indications, contraindications, precautions, dosages, side effects, and the potential adverse reactions.
(5) Provide employee a medication information sheet.
(6) Document all medications received, administered, or discarded.
(7) Post a sign regarding generic versus brand name drugs in the clinic, if required by State laws.
(8) Prepare for emergencies since the potential exists for adverse reactions.
j. Cardiac Emergency Drugs
The cardiac cart must be stocked with emergency drugs
recommended by the American Heart Association (AHA) treatment protocols.
(1) Maintain a list of drugs on the cardiac cart and
postand posted along with treatment protocols.
(2) Maintain an inventory of the drugs and replace drugs prior to the expiration date.
(3) Maintain manufacturer's documentation if drug
expiration date is extended due to shortages.
(4) Locate the cart in an area accessible in the event of an emergency.
(5) Keep cart locked or have an integrity tag in place at all times.
(a) Record lock numbers, when used.
(b) Document the reason locks or integrity tags are replaced.
(6) Make cart readily accessible to all Advanced Cardiac Life Support (ACLS) personnel.
k. Controlled Substances
Based on a needs and risk assessment, there may be a need to stock a limited amount of controlled substances in the clinic.
It is mandatory to comply with State and Federal laws and regulations. A Drug Enforcement Administration (DEA) certificate is required when dispensing or administering controlled substances. A physician must be licensed in the State of practice to obtain the DEA certificate which must be posted with the clinic practice address on it.
(1) Maintain an inventory log showing the drugs received, administered, or disposed.
(a) Include the name and address of the physician, the DEA registration number, date, and time of inventory.
(b) Conduct the inventory with a witness; both physician and witness must sign the inventory.
(c) Retain the inventory and transaction log for a period of 2 years.
(d) Provide the inventory for inspection to the Board of Medical Examiners when requested.
(2) Document each dispensing transaction.
(3) Include the name of the employee, their social security number, name of the drug, quantity prescribed, dosage, date dispensed, the physician prescribing, and the signature of the healthcare provider dispensing the inventory drug.
(4) Store controlled substances in a securely locked, preferably double locked, and substantially constructed cabinet or safe.
(5) Maintain a minimum amount of stock in the inventory.
(6) Restrict access to drugs to key healthcare personnel only.
(7) Report any missing drugs to the DEA, notify the police, Center Medical Director,
(8) Risk Manager, Center Director, Security, and the NASA OHP Medical Director.
l. Immunizations
As a service of the NASA OHP, immunizations are offered to employees to protect them from vaccine-preventable disease. The need for vaccines is based on the employee's occupation, lifestyle, and health status. Assessment of an individual's risk for vaccine-
preventable communicable disease must be made during health maintenance
examinations, medical surveillance examinations, and in preparation for
international travel. Vaccines, such as influenza, may be provided as part of health promotional campaigns. Prior to the administration of vaccines,
clinical guidelines and standing orders must be in place.
(1) Complete a review of immunization history.
(2) Document the immunization history, if not previously documented, and employee's current health status.
(3) Complete an assessment of overall health status
including any possible allergies, existing pregnancy and any immuno-compromised status.
(4) Determine vaccines needed.
(a) Review the vaccine indications, contraindications, precautions, dosages, side effects, and potential adverse reactions.
(b) Ensure completion of informed consent.
(c) Administer vaccine per standing orders utilizing proper aseptic technique.
(d) Document vaccine given, manufacturer, lot number,
location of injection site, date and time given, any reactions, and due
date of next vaccine.
(5) Provide a copy of the Centers for Disease Control (CDC) Vaccine Information Statement (VIS) for the vaccine(s) administered.
(6) Prepare for emergencies since the potential exists for adverse reactions.
(a) Report any adverse reaction to a vaccine to the CDC's Vaccine Adverse Event Reporting System (VAERS).
(b) Send a copy of the CDC VAERS form to the NASA OHP Medical Director.
(7) Store and dispose of vaccines according to the
manufacturer's recommendations.
m. Allergy Injections
The NASA OHP supports the administration of allergy
injections as a convenience for employees. Individual clinic decisions to
offer this level of service should be based on the service volume, adequate
staffing, emergency readiness and willingness to accept responsibility, and
accountability for potential adverse reactions.
(1) Maintain written policy, procedures and standing orders.
(2) Require a written physician's order for allergy
injection administration requests.
(3) Require order to contain employee's name, physician's name, address, and procedures to follow if dosage or timing is missed.
(4) Require employees to receive the first two allergy injections from treating physician.
(5) Require any employee with history of serious or
anaphylactic reaction to see treating physician.
(6) Require a signed an informed consent form prior to beginning injection series.
(7) Store sera in a refrigerator containing only medications.
(8) Utilize safe and aseptic practices when administering injections.
(9) Require employee to remain in the clinic for 20 to 30 minutes for observation.
(10) Require employee resuming allergy injections after a 4-month lapse to receive the first two injections from treating physician.
(11) Prepare for emergencies since the potential exists for adverse reactions.
See also 3.1.4 Handling of Narcotics and Other Regulated Drugs.
n. Emergency Readiness
Emergency readiness is absolutely essential when offering allergy injections, vaccines, and other medications.
(1) Require all staff (e.g. receptionist, and others) to be Basic Life Support (BLS) certified.
(2) Require all nurses and staff administering the vaccines or injections to be ACLS certified.
(3) Require a physician, certified in ACLS, to be present in clinic area when injections or vaccines are administered and during postinjection observation period.
(4) Ensure that emergency procedures are in place and emergency equipment and medications readily available.
(a) Maintain oxygen and epinephrine available in the treatment administration room.
(b) Post emergency telephone numbers near the telephone.
3.1.2.4 References
a. Accreditation Association for Ambulatory Health Care.
b. Executive Order 12564, Drug-Free Federal Workforce.
c. Joint Commission on Accreditation of Healthcare Organizations
d. NASA NPD Control of Narcotics and Other-Regulated Drugs.
e. Physicians Desk Reference 2000.
f. Physicians Desk Reference For Nonprescription Drugs 2000.
a. Physicians Manual: An Informational Outline of the Controlled Substance of 1970.
b. Randolph, Susan A. (1996). Medication Management in the Workplace. AAAOHN Journal, 44(10), 508-512.
c. Rogers, B. Randolph, S. A. and Mastroianni, K. (1996). Occupational Health Nursing Guidelines For Primary Clinical Conditions. Beverly Farms, MA: OEM Press.
j. The Institute for Safe Medication Practices.
3.1.2.5 Flow Diagram
The flow diagram for this process is shown in
Figure 3.1.2 at the end of this section.
3.1.3 Diagnosis and Treatment of Occupational Illness or Injury
3.1.3.1 Introduction
Work-related injury and illness are a leading cause of morbidity and mortality in the labor force and are responsible for decreased productivity and substantial financial costs. Timely diagnosis and treatment of occupational injuries and illnesses are extremely important because they provide an opportunity not only to help the affected employee but also to prevent the recurrence of a similar problem in their coworkers and those in similar jobs.
3.1.3.2 Responsibilities
a. The Medical Director at each NASA Center is responsible for accurate diagnosis and timely treatment of all occupational injuries and illnesses in employees. As the "owner" of this process, this physician is ultimately responsible for initial care, followup, and recovery of the affected employee. Based upon patient history, timeline, and physical findings, the Medical Director will confirm or describe any inconsistencies with a work-related injury or illness. The Medical Director is also responsible for reporting all work-related injuries and illnesses to Center personnel responsible for OSHA recordkeeping.
b. All deaths, work-related or not, will immediately be reported to OHP for forwarding to OCHMO.
c. Any developing trends in diagnosis will be reported to OHP.
3.1.3.3. Process Description
a. The occupational health history is fundamental to the assessment of work-related
, health problems. Additional
, ly,
a total employme
nt history and general health history, including a review of systems and determination of any preexisting conditions are important in achieving an accurate medical diagnosis.
b. The next step should be a complete physical examination with a detailed specific organ or system examination. Laboratory and radiological testing can also be used to complement the history and physical examination and to aid in the diagnosis.
c. Finally, an assessment of the work place by medical and/or safety personnel to enforcesure injury prevention and implementation of approved reasonable accommodation is crucial. All occupational health practitioners should become familiar with employees' work and the environment in which they work. This often necessitates a visit to the workplace and close workings with other areas such as Safety, Workers' Compensation (WC), and Employee Assistance Program (EAP) personnel.
3.1.3.4 References
NASA OHP Web site
3.1.3.5 Flow Diagram
The flow diagram for this process is shown in Figure 3.1.3 at the end of this section.

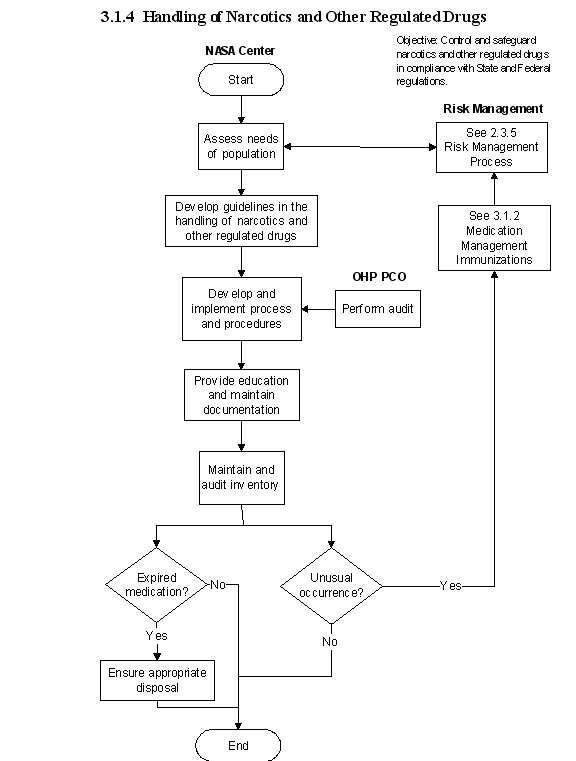
3.1.4 Handling of Narcotics and Other Regulated Drugs
3.1.4.1 Introduction
NASA Occupational Health clinics that store narcotics and other regulated drugs need to have and follow policy/procedures that ensure current regulatory compliance for the handling and storage of such drugs. Regardless of the location of the drugs, whether in medicine cabinets, crash carts or training kits, storage and security criteria must be met. Regulated drugs and medications may be maintained in a variety of locations, based on patient treatment areas and the physical facilities. Any unusual occurrences must be reported immediately to risk management.
3.1.4.2 Responsibilities
a. The Medical Director at each NASA Occupational Health clinic is functionally responsible for compliance with policy/procedures and applicable Federal and State regulations. Additionally all medical personnel are responsible for complying with the policy and regulations.
b. The NASA Centers are responsible for establishing guidelines for the handling of narcotics and other regulated drugs consistent with NASA policies.
c. The Center Drug Inventory Officer shall be a NASA employee, who is neither assigned to the medical staff of the Center's health clinic, nor is the supervisor of the Center's health clinic. The Center Drug Inventory Officer is responsible for maintaining accurate records for controlled drugs, and validating inventory and transaction records at prescribed intervals.
d. The NASA OHP conducts periodic auditing of NASA
Centers' drug policies and procedures.
3.1.4.3 Process Description
Elements of the process include--
a. An assessment of the patient population and potential health risks is the basis for decisions on the appropriate drugs to stock in the clinic setting.
b. A physician is designated responsible for managing the drug program
c. The Medical Director is responsible to for establishing the process and procedures for managing the drugs.
d. The Medical Director provides education to the
healthcare providers responsible for ordering and dispensing of medications.
e. The Medical Director maintains documentation on all drugs procured, dispensed, discarded, or destroyed.
f. The Center Drug Inventory Officer is responsible for establishing, maintaining and auditing the inventory of controlled drugs on a periodic basis.
g. The Center Drug Inventory Officer is responsible for all expired drugs being disposed of according to State and Federal regulations.
h. The Center Drug Inventory Officer is responsible for any unusual occurrences, such as missing drugs or unusual patterns of ordering and for reporting to risk management.
3.1.4.4 References
a. Controlled Substances Act of 1970 (84 Stat. 1242 U.S.C. 801, et seq.1970 Acts Section 704 of Pub.L. 91-513, 84 Stat. 1242, 21 U.S.C. 801 et seq.).
b . See Section 3.1.2, Medication Management, and Appendix D, Medication Management Checklist.
3.1.4.5 Flow Diagram
The flow diagram for this process is shown in Figure 3.1.4 at the end of this section.
3.1.5 Medical Review Officer Drug Free Workplace Program Support
3.1.5.1 Introduction
On September 15, 1988, President Reagan signed Executive Order (EO) 12564, Drug-Free Federal Workplace, which established the goal of a drug-free workplace and made it a condition of employment for all Federal employees to refrain from using illegal drugs on or off duty. The NPR 3792.1, Plan For A Drug-Free Workplace, was developed to implement NASA policy.
3.1.5.2 Responsibilities
a. The DPM ensures that all processes and procedures documented in the NPR are uniformly implemented and conducted throughout the Agency.
b. Reserved.
c. Reserved.
d. A Screening Test Technician (STT) who has training in screening test techniques, may be designated by the Center DPC only after the screening test has been approved for Center use by the NASA DPM and the Agency MRO.
e. The Center Director designates a Drug Program Coordinator (DPC), who is responsible for implementing, directing, administering, and managing the program at the Center and notifies the employee of test results confirmed by the Center MRO.
f. The Center-trained and-certified MRO receives test results directly from the certifying drug test laboratory, confirms positive test results, and reviews them with the Agency MRO prior to notifying the Center. The validity of positive and negative test results is confirmed administratively. Normally, the Center Medical Director is also the Center MRO. If another person is designated as the Center MRO, the Agency MRO must approve the person's credentials.
3.1.5.3 Process Description
a. The AgencyNASA DPM determines the tests needed for the program and which job categories and circumstances in NASA will require drug testing.
b. The Center DPC ensures that drug test sampling at the Center conforms to NASA policies and that collectors of the specimen (Center MRO, if needed) are properly trained to perform their responsibilities.
c. Collections are obtained respectfully, in an evidential manner with Chain of Custody (COC) and confidentiality properly maintained. All positive test results or changes of findings are reviewed by the Agency MRO to confirm validity of findings.
d. Only approved forms and laboratories are utilized. The Agency MRO does not review approved screening tests since the tests only serve to decide if a drug test is indicated. Only test results are reviewed by the MRO.
e. If a nonevidential test is performed, the test must have prior approval for use at a Center by the AgencyNASA DPM and MRO.
f. Audits, maintaining employee confidentiality of MRO test results, and COC completeness records will be available to the Agency MRO and NASA DPM if necessary for Quality Control purposes.
3.1.5.4 References
a. NPR 3792.1, Plan For A Drug-Free Workplace.
b. DG-01NASA, Desk Guide on the Drug-Free Workplace
Program, October 1996.
c. 10 CFR Part 26, Nuclear Regulatory Commission regulations governing Fitness for Duty Programs.
d. 10 CFR Part 121, Federal Aviation Administration/Department of Transportation regulations for Conducting Anti-drug and Alcohol Abuse Prevention Programs for Safety-Sensitive Employees in the Aviation Industry.
e. 49 CFR Part 40, Office of the Secretary of Transportation regulations governing Procedures for Transportation Workplace Drug Testing Programs.
f. 59 FR 29908, Mandatory Guidelines for Federal Workplace Drug Testing Programs, Federal Register, June 9, 1994.
g. EO 12564, Drug Free Federal Workplace, Sept. 15, 1988.
h. SAMHSA, Substance Abuse and Mental Health
Services Administration, Current List of Laboratories Which Meet Minimum
Standards to Engage in Urine Drug Testing for Federal Agencies.
3.1.5.5 Flow Diagram
The flow diagram for this process is shown in Figure 3.1.5 at the end of this section.

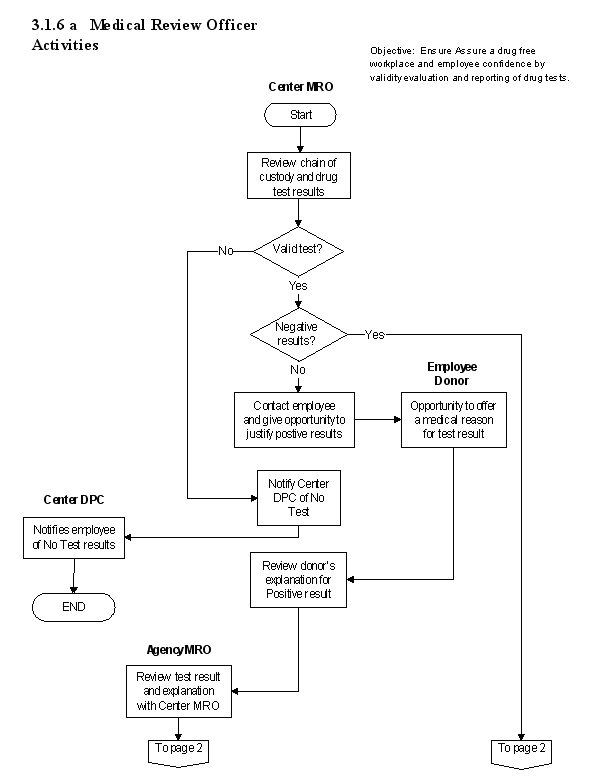
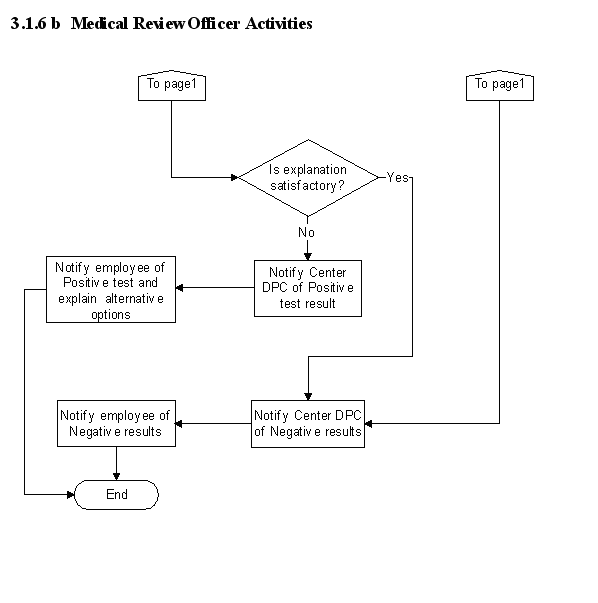
3.1.6 Medical Review Officer Activities
3.1.6.1 Introduction
a. NPR 3792.1A, Plan For A Drug-Free Workplace, defines the MRO roles and responsibilities for NASA. In addition, both the NPR 3792.1A and 49 CFR Part 40, state that "Each Center's MRO must be a licensed physician with knowledge of substance abuse disorders and the
appropriate medical training to interpret and evaluate all positive test
results together with an individual's medical history and any other relevant biomedical information." The Center MRO may be a NASA employee or a contractor for the Agency.
b. Over the years, the accepted medical standard for
qualification has become formal training and certification. The American Association of Medical Review Officers (AAMRO) provide both a training course and certification examinations. The Medical Review Officers' Certification Council (MROCC), is the organization that certifies MRO's. Several States (e.g., Oklahoma in 1994, Florida in 1996) have adopted laws mandating training and certification for MRO's.
c. Reserved.
3.1.6.2 Responsibilities
a. The Center MRO, who is trained and certified confirms positive test results and reviews them and any test result changes with the Agency MRO prior to notifying the Center. The validity of positive and negative test results is confirmed administratively. If another person is designated as the Center MRO, the Agency MRO must approve the person's credentials.
b. The Center Director designates a DPC, who is responsible for implementing, directing, administering, and managing the program at the Center and notifies the employee of test results confirmed by the Center MRO.
c. The NASA Agency MRO develops, implements, and
evaluates EAP and Agencywide medical review functions, including Center MRO
confirmation and negation of test results as it relates to drug and alcohol
testing. The Agency MRO should have additional training as a BAT to qualify for reviewing questions related to EBT's.
d. The NASA Associate Administrator for Human Resources and Education ensures that NPR 3792.1A is implemented, establishes the processes and procedures to carry out the plan, and designates the NASA DPM.
e. The DPM ensures that all processes and procedures documented in the NPR are uniformly implemented and conducted throughout the Agency.
3.1.6.3 Process Description
a. In order for the Center MRO to confirm a result, the following procedures must be completed.
(1) The specimen of the individual selected under Center Drug Program procedures must have been collected properly.
(2) The Chain of Custody (COC) and security of the specimen must have been maintained.
(3) The laboratory testing the specimen must be properly certified.
(4) All test results (positive or negative) must be sent directly to the MRO in a confidential manner.
(5) The specimen must not be adulterated (including
excessive dilution that would interfere with test result) or substituted.
(6) The individual with a positive test result must be given the opportunity to provide a medical reason for a false-positive test.
(7) A positive test result--whether the test remains
positive or is negated as a positive test--must be reviewed with the Agency MRO.
b. Once a determination has been made that the test is a valid test, then the procedures shall be followed.
(1) The MRO-confirmed test result is transmitted to the Center DPC to determine if--
(a) Testing of a prior obtained split specimen is desired by the individual.
(b) If the individual meets program requirements
to obtain a repeat sample if the test nonvalid.
(2) Reserved.
c. If the test is determined to be invalid because of failure to show, failure to provide an adequate collection sample, adulteration, excessive dilution, or failed COC, then the MRO contacts the DPC who determines whether a repeat collection and sample must be obtained under the rules of the program, and the next actions to be taken.
d. If a correctable flaw is found with the COC, the MRO will notify the Center DPC who will assist with obtaining a correction. If the flaw is noncorrectable or "Fatal," the "Test Not Performed" or "Test Cancelled" finding will be marked in Step 8 of the COC form and the DPC contacted.
e. A test may be determined to be positive without having communicated with the employee only under three circumstances.
(1) The employee expressly declines the opportunity to discuss the test. Written documentation of the time and date and statement should be made.
(2) Neither the MRO nor the DPC as the designated employer representative, after making all reasonable efforts, has been able to contact the employee within 14 days of the date on which the MRO receives the confirmed positive test result from the laboratory. DPC assistance should be asked, if no contact with the employee to discuss the
results is made by the MRO after 3 working days.
(3) The DPC as the designated employer representative has successfully made and documented a contact with the employee and instructed the employee to contact the MRO, and more than five days have passed since the date the employee was successfully contacted by the DPC.
f. Reserved.
(1) Reserved.
(2) Reserved.
(3) Reserved.
(a) Reserved.
(i) Reserved.
(ii) Reserved.
(iii) Reserved.
(iv) Reserved.
(v) Researved.
(b) Reserved.
(i) Reserved.
(ii) Reserved.
(iii) Reserved.
(iv) Reserved.
g. If the MRO verifies the test as Positive, the MRO checks the Positive indication with appropriate remarks and reports the result to the employer. If the MRO verifies the test as Negative, the MRO checks the Negative indication and reports the result to the employer. If the laboratory also reports that the specimen was diluted, the MRO reports to the employer that the next time the donor is selected for a drug test, the employer may require the specimen to be collected under direct observation.
3.1.6.4 References
a. NPR 3792.1, Plan For A Drug-Free Workplace.
b. DG-01, NASA, Desk Guide on the Drug-Free Workplace Program, October 1996.
c. 10 CFR Part 26, Nuclear Regulatory Commission regulations governing Fitness for Duty Programs.
d. 10 CFR Part 121, Federal Aviation Administration/Department of Transportation regulations for Conducting
Anti-drug and Alcohol Abuse Prevention Programs for Safety-Sensitive Employees in the Aviation Industry.
e. 49 CFR Part 40, Office of the Secretary of Transportation regulations governing Procedures for Transportation Workplace Drug Testing Programs.
f. 59 FR 29908, Mandatory Guidelines for Federal Workplace Drug Testing. Programs, Federal Register, June 9, 1994.
g. EO 12564, Drug Free Federal Workplace, Sept. 15, 1988.
h. SAMHSA, Substance Abuse and Mental Health Services Administration, Current List of Laboratories Which Meet Minimum Standards to Engage in Urine Drug Testing for Federal Agencies.
i. Memorandum, MRO Guidance for Interpreting
Specimen Validity Test Results, Department of Health and Human Services, Sept 28, 1998, to Medical Review Officers (Certified and Applicant) from Director, Drug and Alcohol Policy and Compliance Office.
3.1.6.5 Flow Diagram
The flow diagram for this process is shown in Figure 3.1.6 at the end of this section.
3.2 Physical Examinations
3.2.1 General Physical Examinations
NASA operations require use of a wide spectrum of physical examinations. The complexity and frequency of these examinations are also quite varied. Some are comprehensive while others may involve only evaluation of a single body system or function. Certain aspects of physical examinations are common to all; section 3.2.1 addresses these generically. Specific and tailored examinations are described in succeeding sections, accompanied by rationale and content. This chapter assembles the basics of most routine and specialty examinations performed at NASA Centers into one procedures chapter.
The expanded listing provided here gives an overview of the many types of physical examinations required in NASA Center operations. Appendix G provides a concise overview in matrix format.
a. General Physical Examinations 95
b. Preplacement Examinations 99
(1) Civil Service Personnel
(2) Pre-Employment Screening
c. Surveillance Examinations 101
(1) Workers with Specific Potentially Hazardous Exposures
(a) Arsenic
(b) Asbestos
(c) Benzene
(d) Beryllium
(e) Cadmium
(f) Chromium
(g) Ethylene Oxide
(h) Formaldehyde
(i) Lead
(j) Mercury
(k) Methylene Chloride
(l) Methylene Diisocyanate, Methylene Triisocyanate
(m) 4,4'-Methylene Bis (2-Chloroaniline) and 4,4'-Methylene Dianiline
(n) Phenylenediaminie
(o) Nickel
(p) Polychlorinated Biphenyls
(q) Thermal Ablative, Methylene Biphenyl Isocyanate
(r) Trichloroethylene
(s) Tungsten Carbide
(t) Noise (Hearing Conservation)
(u) Silica Dusts
(2) Workers in Potentially Hazardous Environments
(a) Chemical Laboratory Workers
(b) Hazardous Waste Workers
(c) Hearing Conservation
(d) Spray Painting
(e) Water and Sewage
d. Job Certification Examinations 115
(1) Air Traffic Controller
(2) Confined Space/Tank Entry
(3) Crane Operator/Ground/Floor/Remote Operated Cranes
(4) Crane Operator/High
(5) Diver
(6) Down Range/Shipboard and Remote Assignments
(7) Federal Aviation Administration Personnel
(8) Fire Fighter
(9) Food Handler
(10) Fuel Handler/Contingency Crew
(11) Hazardous Materials Emergency Response Team
(12) Heavy Ordinance
(13) High Crew/Spider
(14) Locomotive Engineer/Crawler-Transporter
(15) Motive (Heavy) Equipment Operators
(16) Multiple Passenger Vehicle Operator
(17) Department of Transportation Commercial Drivers
License
(18) Motor Vehicle Certification
(19) Non-Crew Flying
(20) Self Contained Atmospheric Protective Ensemble (SCAPE)
(21) Security Personnel
(22) Solid Rocket Booster Retrieval
e. Special-Purpose Examinations 127
(1) Employment Situations
(a) Disability Retirement
(b) Fitness for Duty - General
(c) Fitness for Duty - Civil Service
(d) Fitness for Duty - Contractor
(e) Return to Work
(2) Visual Examinations
(a) General Examinations
(b) Console Color spectrum
(c) Dye Penetrant
(d) Microscopic Particle Counting
(e) Solderers and Welders
(3) Workplace Exposures
(a) Bloodborne Pathogens
(b) Laser Workers
(c) Primary Animal Contact
(d) Primary Crew Contact
(e) Ionizing Radiation Workers
(f) Respirator, Occupational (Non-SCAPE)
(g) Respirator, Other
(h) Tuberculosis Control
f. Health Maintenance Examinations 138
(a) Federal Employees Health Program
(b) NASA Executives
(c) Key Contractor Executives
3.2.1 General Physical Examinations
3.2.1.1 Introduction
a. Reserved.
b. Physical examinations are performed for several purposes. Local, state or federal regulations may direct physical examinations for certain categories of employees. The employer, employee, or an examining physician may also request examinations. They may also be a part of the employer's standard operating procedures. Regardless of the reason for initiating the examination, the purpose is to protect the health and well-being of employees and coworkers, and to ensure their optimal work performance.
c. Examinations are structured to determine if the employee can safely and adequately accomplish the essential functions of a job, to determine if performance of the job has produced any evidence of an effect on the employee's health, or to monitor the general health of the employee. A single examination may serve several purposes. Results of the examinations are communicated to the employee and the employer, and are made available to the employee's personal physician within the strict guidelines on confidentiality and Code of Ethical Conduct.
d. Regarding currency of an examination, if a physical examination has been conducted within the previous six months and has been duly recorded in the employee's health record, it may, at the discretion of the examining physician, be accepted in whole or in part as the requested medical examination. Complete examinations conducted more than 6 months previously may be utilized with appropriate supporting information and a signed interval note by the cognizant examining
physician. A physical examination conducted for one purpose is valid for any other purpose within the prescribed validity period if that physical contains the proper data. If the examination is deficient in scope, only those tests and procedures needed to meet the additional requirements are performed. The results are recorded and the examining physician signs the appropriate approval.
e. Physical examinations are grouped into five categories: Preplacement, Surveillance, Job Certification, Special Purpose, and Health Maintenance.
3.2.1.2 Responsibilities
a. Managers and supervisors identify, with occupational medical, environmental health, and safety consultations, as appropriate, which jobs require any level of medical evaluation, if hazards
exist to workers, job certification and employee medical surveillance are indicated. Special-purpose and health maintenance examinations will be required at the discretion of Senior Management according to corporate policy and other regulatory guidelines.
b. Reserved.
c. All disciplines required in health care delivery are responsible in their areas of expertise for accomplishing and interpreting medical evaluations.
d. Appeal, redress, second opinions, and challenged
decisions will be handled at the lowest level of authority. The NASA Medical Policy Board holds final decision authority in contested situations.
3.2.1.3 Process Description
a. The following recommended intervals are given for those situations where there are no established frequency guidelines. These recommendations are not intended to take precedence over Center requirements.
|
Type
|
Frequency
|
|
Preplacement
|
As required
|
|
Surveillance
|
Annually unless otherwise stated
|
|
Job Certification
|
Age-determined unless otherwise stated
|
|
Health Maintenance
|
Age-determined unless otherwise stated
|
|
Special-Purpose
|
As required
|
|
Age
|
Frequency
|
|
Up to age 40
|
Every 5 years (ages 20, 25, 30, 35, 40)
|
|
Age 40-50 years
|
Every 2 years (ages 42, 44, 46, 48, 50)
|
|
Over age 50
|
Annually
|
b. A complete physical examination is to be performed in all preplacement and initial surveillance examinations, and in most job certification and health maintenance examinations.
c. Reserved.
d. Every complete physical examination includes (unless the employee declines) the following:
(1) Disrobing
(2) Head, Eyes, Ears, Nose and Throat (HEENT), including ophthalmoscopic
(3) Lymphatic
(a) Cervical
(b) Axillary
(c) Inguinal
(4) Pulses
(a) Carotid
(b) Radial
(c) Dorsalis pedis/posterior tibial
(5) Auscultation of heart, lungs, and abdomen.
(6) Abdominal palpation and inspection.
(7) Inguinal hernia examination.
(8) Scrotum and contents examination.
(9) Inspection of extremities.
(10) Rectal and prostate examination at age 40 and over are performed with the physical examination, unless the employee testifies to examination by a private physician within 1 year.
(11) Hemoccult offered in accordance with the American College of Surgeons (ACS) recommendations.
(12) Female breast examinations are offered.
(13) Females are advised regarding the best available current mammography recommendations and strongly encouraged to have appropriate studies.
(14) Pelvic examination and Papanicolaou (Pap) tests are offered to those female employees in the Federal Employees Health Program (FEHP), active duty military service, and the executive physical examination program. This examination may not be needed if the employee testifies to a normal examination by a private physician within the previous year.
(15) Additionally, documentation of the completed examination includes--
(a) Annotation to reflect positive or negative findings relevant to items raised by the history.
(b) Comments on all abnormal findings.
(c) Record of diagnosis.
(d) Record of recommendations.
3.2.1.4 References
a. Centers for Disease Control (CDC) "Blue Sheet" (Weekly summary of countries with infected areas of Quarantined Disease and Health Information for International Travel).
b. Federal Aviation Administration (FAA) Guide for Aviation Medical Examiners.
c. Federal Personnel Manual Supplement 339-31, Reviewing and Acting on Medical Certificates.
d. 29 CFR Part 1910.120, Occupational Safety and Health Administration, 29 regulations governing Hazardous Water Operation and Emergency Response.
e. Respirator Medical Evaluation Questionnaire.
f. Refer also to Appendix G for a detailed matrix of all examinations described in sections 3.2.2 through 3.2.6.
3.2.2 Preplacement Examinations
3.2.2.1 Introduction
Preplacement physical examinations are conducted to determine if an employee can safely and adequately perform the essential functions of a job. The essential functions of a job are identified by the employer and communicated to the examining physician. Preplacement examinations are performed before assignment to the job. (The standards for NASA and other civil service personnel are outlined in the Federal Personnel Manual Supplement 339-31, Reviewing and Acting on Medical Certificates. The physical requirements for the job are specified on the Standard Form (SF) 88, which is completed at the time of the examination.)
3.2.2.2 Reserved.
a. Reserved.
(1) Reserved.
(2) Reserved.
(a) Reserved.
(b) Reserved.
(c) Reserved.
(i) Reserved.
(ii) Reserved.
(iii) Reserved.
(iv) Reserved.
(3) Reserved.
b. Reserved.
(1) Reserved.
(a) Reserved.
(b) Reserved.
(2) The pre-employment examination includes the following:
(a) Occupational and medical history. (Utilize questionnaires where applicable.)
(i) Document past occupational exposures to chemical and physical hazards
(ii) Document past illnesses and chronic diseases such as atopy, asthma, lung diseases, renal and cardiovascular problems.
(b) Review symptoms, respiratory, high blood pressure.
(c) Identify employees who are vulnerable to particular substances.
(d) Document lifestyle habits such as smoking, alcohol and drug abuse.
(3) A complete physical examination is performed to identify conditions that may place the employee at risk. Examples are obesity, lack of conditioning, respiratory problems such as chronic obstructive pulmonary disease, facial abnormalities, and orthopedic
problems such as severe arthritis, missing digits.
(4) Laboratory data.
(a) Sequential Multiple Analyzer Computer (SMAC) blood chemistry profile, Complete Blood Count (CBC), Urinalysis (U/A), Pulmonary Function Test (PFT), Electrocardiogram (ECG), vital signs, visual testing, audiometric testing, Chest X-ray (CXR).
(b) Pertinent baseline biomonitoring as appropriate,
e.g., Blood Lead Level (BLL) and Zinc Protoporphyrins (ZPP) for lead workers; Cadmium in Blood (CdB), Cadmium in Urine (CdU) and Beta-2 Microglobulin in urine (B2-M) for cadmium workers; Polychlorinated Biphenyl (PCB) levels for PCB workers; and acetylcholinesterase levels for pesticide workers.
3.2.3 Surveillance Examinations
3.2.3.1 Introduction
Identification of workers needing specific
surveillance examinations is the responsibility of the employer. The examination may be for only one chemical exposure or a category of exposures. The extent of the "hands-on" examination, laboratory and special procedure examinations required for each category of surveillance physical is specified in writing. If the examinations are not performed onsite, the OHP Medical Director reviews the results before clearance is issued to work in a hazardous environment.
3.2.3.2 Process Description
This section provides guidance for treating workers with specific potentially hazardous exposures.
a. Arsenic
(1) Reference: OSHA 29 CFR 1910.1018
(2) Frequency:
(a) Preplacement for all employees who are or may be exposed to arsenic at or above the action level 30 or more days per year.
(b) Semiannually for employees 45 years old or older or 10 or more years of exposure over the action level.
(c) Annually for all other covered employees.
(d) Ad hoc examination if for any reason the employee develops signs or symptoms usually associated with exposure to inorganic arsenic.
(e) Termination if no examination has occurred within the past 6 months.
b. Asbestos
(1) Reference: OSHA 29 Part CFR 1910.1001 and 29 CFR Part 1926.1101.
(2) Frequency: Preplacement, annually and termination for asbestos workers.
(3) Medical evaluations and physical examinations are offered to employees exposed or potentially exposed to asbestos fibers in accordance with regulations listed above.
(4) Evaluation of asbestos exposure status consists of the following:
(a) Completion of the Occupational Health Questionnaire for Asbestos Workers (available in Appendix D, parts 1 and 2 of the OSHA Asbestos Standard) to include a detailed occupational history with special emphasis on exposure to dusts, fumes, fibers, gases, and any other respirable materials or compounds.
(b) History of respiratory system disease of any nature, e.g., infections, allergies, congenital abnormalities.
(c) Smoking history.
(d) History of other household members exposed to asbestos fibers.
(5) Incidentally exposed employees are offered a complete baseline examination, but no periodic examination is scheduled.
(Incidentally exposed are those people who may have been exposed in excess of the action level on a one-time basis. EH personnel can assist to validate.)
(6) Civil service personnel requiring asbestos evaluation and/or surveillance may have these procedures combined with their FEHP examination.
(7) All findings, negative as well as positive, are noted in the medical record. The latter are identified and retained in
accordance with 29 CFR 1910 asbestos regulations.
(8) Current CXR surveillance schedule is as follows:
(a) Baseline/preplacement Posterior-Anterior (PA) chest.
(b) Periodic PA chest schedule--
(1) 1-10 years since first exposure: CXR every 5 years.
(2) 10+ years since first exposure:
(3) Age 35: CXR every 5 years.
(4) Age 35-45: CXR every 2 years.
(5) Age 45+: CXR annually.
(6) Termination CXR in accordance with the periodic schedule.
(c) Only asbestos workers who have been exposed to airborne asbestos in excess of the action level or the excursion limit are medically monitored.
c. Benzene
(1) Reference: OSHA 29 CFR Part 1910.1028.
(2) Frequency: Preplacement, annually and ad hoc for employees exposed to airborne benzene at or above the action level, TWA, or STEL as applicable to the time spent working at these levels.
d. Beryllium
(1) Reference: OSHA 29 CFR Part 1910.1000.
The International Agency for Research on Cancer concluded in 1993 that "there is sufficient evidence in humans for carcinogenicity of beryllium and beryllium compounds."
(2) Frequency: Preplacement and annually for employees exposed at or above an action level.
e. Cadmium
(1) Reference: OSHA 29 CFR Parts 1910.1027 and 1926.1127.
(2) Frequency: A complete initial medical examination occurs within 30 days after the initial medical examination includes biological and medical monitoring. Each request for cadmium surveillance physical examination is validated by EH personnel. Medical
surveillance is required for any employee who is, or may be, exposed at or
above the airborne action level for 30 days or more per year. The frequency of monitoring initial assignment to a job with exposure to cadmium above the airborne action level depends upon the biomonitoring
category as defined in the following sections:
(a) Biological monitoring includes SMAC, CBC, U/A, CdU, CdB, and B2-M. Biological monitoring occurs at least annually.
(b) Medical monitoring occurs with the initial examination at 12 months and at least every 2 years thereafter. The medical monitoring examination is performed by an OHP physician, or a health care professional designated by the physician, and includes the following in addition to the biologic monitoring:
(i) Baseline CXR (physician determines CXR frequency on an as need basis after baseline).
(ii) Detailed history, to include medical and work history, smoking status, reproductive history, nephrotoxic medication usage, any history of renal, cardiovascular, respiratory, hematopoietic,
and/or musculoskeletal dysfunction.
(iii) Pulmonary function testing.
(iv) Physical examination to include prostate palpation for all males over 40 years of age, blood pressure, and Prostate Specific Antigen (PSA) testing for males over age 50.
(3) A medical examination is performed as soon as possible whenever an employee demonstrates difficulty in breathing during a
respirator fit test, or during use of a respirator.
(4) The exit cadmium physical examination includes a CXR.
(5) For each medical examination, a physician-signed written medical opinion is sent to the employer. The employer is required to forward these data to the employee within 2 weeks of receipt. The written medical opinion includes the following:
(a) Any diagnosis related to cadmium exposure.
(b) Any detected health risks from cadmium exposure.
(c) Results of biological monitoring.
(d) Recommended removal or duty limitations.
(e) A statement that the physician has clearly and carefully explained to the employee the results of the medical examination, including all the biological monitoring results and any medical conditions related to cadmium exposure that requires further evaluation or treatment, and any limitations on the employee's diet or use of medications. The medical opinion does not include any findings or diagnoses unrelated to occupational exposure to cadmium.
(6) The biological action levels and biomonitoring
categories will be dictated by current OSHA standards.
(7) All medical removals occur with the concurrence of the OHP Medical Director. Medical removal is at the discretion of the physician when exposure is between normal and "high triggers." Above the "high triggers," mandatory removal occurs. The employer is responsible for removing the employee from exposure to cadmium above the airborne action level as soon as possible after written notification from the examining physician has been received. The employee has certain Medical Removal Protection (MRP) rights as outlined by OSHA regulations.
(8) Records Retention: Records are retained for employment period plus 30 years.
f. Chromium
(1) Reference: OSHA 29 CFR Part 1910.1000
(2) While there are no OSHA, NASA, or State requirements for a chromium or chromic acid medical surveillance examination, special situations may exist when periodic surveillance examinations are advisable.
g. Ethylene Oxide
(1) Reference: OSHA 29 CFR Part 1910.1047
(2) Frequency: Preplacement, annually, and ad hoc when specified ethylene oxide worker is exposed at or above the action level.
h. Formaldehyde
(1) Reference: OSHA 29 CFR Part 1910.1048
(2) Frequency: Preplacement, annually, and ad hoc for employees exposed at or above the action level or exceeding the STEL.
i. Lead
(1) Reference: OSHA 29 CFR Parts 1910.1025 and 1926.62
(2) A medical surveillance program is provided by NASA for employees who are or may be exposed to an airborne concentration of lead at or above an OSHA action level. The medical surveillance program is under the direction of the OHP Medical Director and complies with the requirements established in the OSHA Lead Standards referenced above.
(3) NASA is responsible for identifying employees who may require enrollment in medical surveillance program. This information is provided to OHP medical personnel via the appropriate form notification. The employee is enrolled in the medical surveillance program, and the appropriate EH personnel determine if the employee meets the OSHA requirements for continued enrollment. The medical surveillance program includes biological monitoring and medical examination or consultation.
(4) Biologic monitoring consists of blood sampling for BLL and ZPP. The frequency of biologic monitoring is every 2 months for the first 6 months and every 6 months thereafter.
(5) A complete physical examination is performed as the initial preplacement examination and at least every 2 years. This frequency may be more often than 2 years, if deemed necessary by the examining physician.
(6) NASA makes an initial medical surveillance examination available to employees who are occupationally exposed on any day to an airborne lead concentration at or above the OSHA action level.
This initial medical surveillance includes blood sampling for BLL and ZPP.
The proper OSHA criteria have to be met, as determined by the appropriate EH personnel.
(7) The BLL action level will be dictated by current OSHA standards.
(8) OHP medical personnel forward all BLL values to NASA. NASA is required to provide a copy of the employee's BLL to the employee within 5 working days after the employer's receipt of the BLL results.
(9) A medical examination occurs if the BLL exceeds the action level. EH personnel are notified by OHP medical personnel to begin an evaluation of the employee's work practices and other aspects of lead exposure, if the BLL action level is met or exceeded. The OHP Medical Director or a physician designee performs the medical evaluation within a 2-week period after NASA receives notification of the elevated BLL. The BLL is repeated at the time of the medical evaluation. A medical examination also occurs as soon as possible on the following:
(a) After an employee develops or complains of signs and symptoms of lead intoxication,
(b) After an employee desires medical advice concerning the effects of current or past exposure to lead on the employee's ability to procreate a child, and
(c) After an employee, who is enrolled in the medical surveillance program, has demonstrated difficulty in breathing during a respirator fit test or during use, or
(d) After it is known that the employee is pregnant.
Any time employees have a medical examination mandated for any of the above reasons, they are notified by NASA of their guaranteed right to seek a second medical opinion from another physician of the their choice, regarding the occupational exposure to lead. Any discrepancy or dispute between the second physician and the initial evaluating physician is settled in consultations/negotiations or by a third physician.
(10) The medical examination contains a detailed work history, medical history, recording of possible past lead exposures, personal habits (smoking and hygiene), past gastroenterological, renal, cardiovascular, reproductive, or neurological problems. A physical examination is performed with attention to the teeth, gums, hematologic, gastrointestinal, renal, cardiovascular, pulmonary, and neurological status. Blood pressure, BLL, ZPP, and laboratory profile (to include hemoglobin, hematocrit, red cell indices, peripheral smear morphology,
blood urea nitrogen/Cr, U/A with micro) are required. If requested by the
employee, pregnancy testing and laboratory evaluation of male fertility is
performed.
(11) Removal (MRP) of an employee from a worksite where lead exposure can occur at or above the OSHA airborne concentration of lead action level is mandatory if the BLL exceeds OSHA removal limits. NASA is responsible for removing the employee from lead exposure as soon as possible after the notification from OHP medical personnel is received. Once removed from a lead work environment due to an elevated BLL, the employee is permitted to return to work only after clearance is received from the OHP physician. BLL values are determined at least every month until it is determined that the employee can safely return to work. Clearance is given when two consecutive BLL values are at or below established levels and return to work is not medically contraindicated.
(12) The OHP physician may recommend removal of any
employee from a worksite where the airborne concentration of lead exceeds the OSHA action level. This is done if a medical condition detecte in the employee, or if there is concern that a medical finding, determination, or opinion, would place the employee at increased risk of material impairment to health from exposure to lead.
(13) For each medical examination that requires employee removal from any occupational exposure to lead above the OSHA action level, a written statement is created by the examining physician and provided to the employee in accordance with the Privacy Act. The physician's opinion statement contains at least the following:
(a) Any detected medical condition that would place the employee at increased risk of material impairment of the employee's health from exposure to lead.
(b) Any recommended special protective measures or
limitations to be placed upon the employee's exposure to lead.
(c) Any recommended limitation upon the employee's use of respirators, including a determination of whether the employee can wear a powered air purifying respirator or if a physician determines that the employee cannot wear a negative pressure respirator.
(d) The results of the BLL determination.
(14) The written opinion does not reveal any findings or diagnoses unrelated to an employee's occupational exposure to lead. However, the physician, either verbally or in writing, advises the employee of any medical condition (and properly records this advice in the medical chart), occupational or nonoccupational, which dictates further medical examination or treatment.
(15) Employees who are determined not to be medically qualified for work according to lead exposure requirements are to have a medical opinion statement sent directly to them in accordance with the Privacy Act. OHP medical personnel also inform the employer of the
employee's status as appropriate for the circumstances.
j. Mercury
(1) Reference: Agency for Toxic Substances and Disease Registry (ASTDR), 1992
(2) Frequency: Complete preplacement examination, with annual laboratory screening. Complete physical examination at least every 2 years.
(3) Laboratory: SMAC, CBC, U/A, visual testing, PFT, 24-hour urine mercury.
k. Methylene Chloride
(1) Reference: OSHA 29 CFR Part 1910.1052
Medical surveillance is provided for all employees who are or may be exposed to Methylene Chloride (MC), at or above the action level for 30 or more days per year. It is also provided for employees who are exposed at or above the Permissible Exposure Limit (PEL),
or STEL for 10 days or more days during the year. Medical surveillance is also provided for any employee exposed above the PEL or STEL, who has been identified by a physician or other licensed health care professional as at risk for cardiac disease or some other serious MC-related health condition. Any employee may request inclusion, regardless of the duration of MC exposure. Medical surveillance is also available to all employees exposed to MC during an emergency.
(2) Frequency: The medical examination surveillance consists of a preplacement examination and annual examinations
thereafter.
(3) Contents of the medical examination will include the following:
(a) A comprehensive medical and work history. This history includes an annual detailed work and medical history with special emphasis on cardiac history, skin conditions, history of hematologic
disorder, liver disease, and MC exposures, work practices, and personal
protective equipment used. Information should also be obtained from the worker regarding potential signs or symptoms associated with increased levels of carboxyhemoglobin. The examiner ensures that the smoking history of all employees exposed to MC is known.
(b) A complete physical examination with special emphasis on the lungs, cardiovascular system, liver, nervous system, skin, and vital signs. An evaluation of the employee's ability to wear a respirator.
(c) Laboratory surveillance: SMAC, CBC, U/A, ECG, PFT, visual testing.
(4) The OHP physician gives the affected employee a written opinion regarding results of the examination within 15 days of completing the evaluation of the medical and laboratory findings, but no more than 30 days after the initial examination. NASA receives notification that such written medical opinion has been provided to the employee. The written medical opinion is limited to the following:
(a) The physician's opinion as to whether the
employee has any detected medical conditions that would increase the risk of material impairment from exposure to MC.
(b) Any recommended limitations on employee exposure to MC and on the use of personal protective clothing or equipment and respirators.
(c) A statement that the physician has informed the employee that MC is a potential occupational carcinogen, of the risk factors for heart disease, and the potential exacerbation of underlying heart disease from MC exposure and its metabolism to carbon monoxide.
(d) A statement that the physician has informed the
employee of medical examination results and any medical conditions resulting from MC exposure requiring further explanation or treatment.
(5) The physician must not reveal to the employer, orally or in writing, any specific records, findings, or diagnoses that have no bearing on occupational exposures to MC.
(6) MRP benefits are required for employees removed from work exposure to MC, or for whom limited exposure to MC is recommended, due to existing/potential medical problems. MRP benefits are
required if the physician finds that exposure to MC may contribute to or
aggravate the employee's existing cardiac, hepatic, neurological or dermal symptoms.
, ,
(7) Medical Records
(a) Will be kept for the duration of the employee's employment plus 30 years.
(b) In addition to demographics, the record contains the physician's written opinion and employee medical conditions related to MC exposure.
l. Methylene Diisocyanate, Methylene Triisocyanate
(1) Frequency: Complete preplacement physical
examination and annual laboratory screening. Complete physical examinations a minimum of every 2 years.
(2) Laboratory: SMAC, CBC, U/A, PFT, visual testing.
m. 4, 4'-Methylene Bis (2-Chloroaniline) and 4,4'-Methylene Dianiline
(1) Reference: OSHA 29 CFR Part 1910.1050, 29 CFR Part 1926.62 (MDA) ASTDR Toxicological Profile on 4,4'-Methylene Bis (2-Chloroaniline)
(2) Frequency: Complete preplacement physical
examination and annual laboratory. Complete physical examination a minimum of every 2 years.
(3) Laboratory: SMAC, CBC, U/A, visual testing, PFT, urine analysis for chemical content, and urinary cytological examination.
n. M-Phenylenediamine
(1) Frequency: Complete preplacement physical
examination and annual laboratory screening. Complete physical
examinations a minimum of every 2 years for workers exposed at or above the action level for 30 or more days per year.
(2) Laboratory: SMAC, CBC, U/A, PFT, visual testing.
o. Nickel
(1) Reference: NIOSH 77-164 1977
Inorganic nickel causes allergic eczema and probably is a cause of respiratory cancers. Soluble nickel compounds are irritants to the eyes, skin, and respiratory tract.
(2) Frequency: Complete preplacement physical examination and annual laboratory screening. Complete physical examinations a minimum of every 2 years for workers exposed at or above the action level for 30 or more days per year.
(3) Laboratory: SMAC, CBC, U/A, PFT
p. Polychlorinated Biphenyl
(1) Reference: NIOSH 77-225 1977
(2) Frequency: Complete preplacement
examination and annual blood testing for serum PCB's as a minimum if there is a history of exposure, and ad hoc if significant exposure is documented or suspected.
(3) Special emphasis on hepatic and dermatological findings. Special work history for hazardous environments.
q. Thermal Ablative, Methylene Biphenyl Isocyanate
(1) Frequency: Complete preplacement examination and annual laboratory screening. Subjective and/or objective evidence of exposure requires complete examination. Complete examination at least every 2 years.
(2) Evaluation includes general occupational medicine history with special attention to the following:
(a) Previous exposure to isocyanates and other
industrial chemical irritants.
(b) History of broncho/pulmonary disease or
hypersensitivity.
(c) History of chronic eye or skin condition or hypersensitivity.
(d) History of anemia or other blood dyscrasia.
(e) Liver disease.
(f) Pulmonary function (forced vital capacity,
forced expiratory volume in 1 second).
(g) CXR.
(h) Eye and vision examination with special attention given to cornea and conjunctiva.
(i) Chemistry battery with special attention to
liver function tests.
(j) CBC.
(k) Urinalysis.
(l) Physical examination with special attention to evaluation of skin, lungs, liver, and spleen.
r. Trichloroethylene
(1) Trichlorethylene is classified as a probable human carcinogen.
(2) Employees having exposures above the action level for 30 or more days per year are included in the medical surveillance program.
s. Tungsten Carbide
Tungsten carbide tools used for cutting, drilling, sawing, and grinding contain cobalt. Dusts from this alloy can cause skin irritation and a variety of respiratory conditions. Employees assigned to work on a regular schedule with these devices are included in the medical surveillance program.
t. Noise (Hearing Conservation)
(1) Reference: OSHA 29 CFR Part 1910.95.
(2) The employee completes an appropriate baseline hearing questionnaire.
(3) Physical examination of the ear, nose, and throat system is accomplished prior to audiometry as deemed appropriate.
(4) An Audiogram is appropriately recorded.
(5) The examiner evaluates findings, past records, and recommends appropriate actions in accordance with dictates of good occupational health practice.
(6) Referrals for audiologic/otologic consultation are reviewed and approved by the OHP Medical Director or his designee prior to implementation.
(7) Hearing Evaluation Report Form Disposition and Hearing Conservation Registry:
(a) The original form is filed in the employee's medical record as a physical examination.
(b) The primary Hearing Conservation Registry consists of an alpha file of individuals.
(c) The annual hearing examinations are filed in individual employee's medical records.
(8) Each employee with a significant threshold shift is evaluated by a physician, preferably the physician overseeing the hearing conservation program.
(9) Each employee receives a letter detailing the results of the audiometric examination.
u. Silica Dusts
Crystalline or free silica inhalation may cause silicosis and is a mechanical irritant to the skin and eyes. It is categorized as a probable human carcinogen. Medical surveillance is recommended for employees exposed to crystalline silica at or above the action level for 30 or more days per year.
This section provides guidance for treating workers in potentially hazardous environments as detailed below.
a. Chemical Laboratory Workers
(1) Reference: OSHA 29 CFR Part 1910.1450.
This recommendation covers medical surveillance for workers having occupational exposure to hazardous chemicals in chemical laboratories. The laboratory is a workplace where protective laboratory practices and equipment are in place and relatively small quantities of hazardous chemicals are used on a nonproduction basis. This recommendation includes workers wearing "splash" protection that is not included in other physical examination category. It does not include workers in medical clinical laboratories.
(2) Frequency: Annually when employees are exposed to any specific regulated substance above the action level for 30 or more days per year, or if no action level is established, the PEL for 30 or more days per year.
b. Hazardous Waste Workers
(1) Reference: OSHA 29 CFR Parts 1910.120 and 1926.65.
(2) Frequency: Employees who are exposed to hazardous materials at levels greater than the action level for more than 25 days per year, who wear respiratory protection, or who have a significant potential for exposure through skin absorption, are provided initial, periodic, and terminal medical surveillance. Employees who may be covered by this program are employees involved in the transport, storage, and disposal of hazardous substances. This category also includes employees assigned to support hazardous operations other than those supported by the Hazardous Materials (HAZMAT) emergency response team. (The latter must also meet firefighter standards.) Some of these hazardous substances are as follows:
(a) Aromatic hydrocarbons.
(i) Benzene.
(ii) Ethyl benzene.
(iii) Toluene.
(iv) Xylene.
(b) Asbestos (or asbestiform particles).
(c) Halogenated aliphatic hydrocarbons.
(i) Carbon tetrachloride.
(ii) Chloroform
(iii) Ethyl bromide
(iv) Ethyl chloride
(v) Ethylene dibromide
(vi) Ethylene dichloride
(vii) Methyl chloride
(viii) Methyl chloroform
(ix) Methylene chloride
(x) Tetrachloroethane
(xi) Tertachloroethylene (perchloroethylene)
(xii) Trichloroethylene
(xiii) Vinyl chloride
(d) Heavy metals.
(i) Arsenic
(ii) Beryllium
(iii) Cadmium
(iv) Lead
(v) Mercury
(e) Herbicides.
(i) Chlorophenoxy compounds.
(ii) Dioxin
(f) Organochlorine insecticides.
(i) Chlorinated ethanes
(ii) Cyclodienes: aldrin, chlordane, dieldrin, endrin
(iii) Chlorocyclohexanes: lindane.
(g) Organophosphate and carbamate insecticides.
(i) Organophosphate: diazinon, dichlorovos, dimethoate, trichlorfon, malathion, methyl parathion, parathion.
(ii) Carbamate: aldicarb, baygon, zectran.
(h) Polychlorinated biphenyls.
(3) The medical surveillance program includes a
complete physical and biological monitoring with pre-employment screening and periodic medical examinations as appropriate for the specific
substances involved.
c. Insect and Pest Control
(1) Frequency: Complete preplacement examination, annual laboratory screening for pesticides and herbicides (bone marrow, liver, renal) and at least biennial physician examination. (See
requirements in Section 3.2.3.2 concerning Hazardous Waste Workers.)
(2) Red blood cell cholinesterase is performed at least annually. (Recommended more frequently if handling organophosphates or carbamate pesticides on a regular basis.)
d. Spray Painting
Spray painters may encounter several hazardous chemicals. These include cadmium, chromium, lead, isocyanates, acrylates, organic solvents, epoxies, and glycol ethers. There may also be physical hazards such as heat, ergonomic stresses, working at heights, noise, and dusts.
e. Water and Sewage
(1) Frequency: Complete preplacement examination and annual laboratory screening.
(2) Immunizations may be provided on a voluntary basis but are not required.
(a) Typhoid - every 3 years.
(b) Tetanus/Diphtheria - every 10 years.
(c) Hepatitis A.
3.2.4 Job Certification Examinations
3.2.4.1 Introduction
Job certification examinations may be required by Federal or State statutes or by the employer. The Agency or organization establishing the requirement specifies the medical, laboratory and special procedure standards for these examinations. If the examinations are not performed onsite, the OHP Medical Director reviews the results before certifications are issued for doing the job.
3.2.4.2 Process Description
(1) The applicable medical standards for air traffic control are found in the FAA Guide for Aviation Medical Examiners (AME).
(2) There are special concerns when providing health care to air traffic control employees. A physician with special training in aerospace medicine (either experience as a military or civilian flight surgeon or as an FAA AME, or training in military or civilian aerospace medicine residency programs) should always see these employees.
(3) Two general concerns apply for air traffic controllers. The first is that a medical condition itself may prevent them from safely being able to fulfill their duties. The second concern is that the treatment for a condition, even though minor, may interfere with their ability to perform their normal duties. Air traffic controllers are not to control aircraft when administered medication that may affect their alertness or mental process.
(4) Air traffic controllers are advised that they are not to perform these duties when being administered antihistamines, decongestants, tranquilizers, antidepressants, muscle relaxants, or sleeping medicines.
(5) When a health care worker, other than a qualified aeromedical physician, sees an air traffic controller, the OHP Medical Director is notified and requested to recommend appropriate treatment and disposition. Documentation is maintained in the medical record and two copies given to the employees for their supervisor and for their own record. If it is appropriate that the employees can return to work, but not do air traffic controller duties, then their duty section or supervisor is notified. Employees are also told that they have to see OHP medical personnel for release to full duty when they are medically qualified.
(6) All information related to complaints, physical examination findings, diagnosis, treatment, and disposition is recorded in the employee's medical record. If there is uncertainty with regard to disposition, the OHP Medical Director is consulted.
b. Confined Space/Tank Entry
(1) Frequency: Initial complete physical and age determined thereafter. Concomitant chemical or physical exposures may dictate that surveillance physicals also be performed.
(2) Particular attention by the examining physician is directed to HEENT, neurological examination, and history to include
organic conditions predisposing to syncope, vertigo, convulsions.
(3) Review of baseline laboratory data specific for detecting organic effects of the particular chemicals encountered.
(4) Subject should be free of psychoemotional instability.
c. Crane Operator/Ground/Floor/Remotely Operated Cranes
(1) Frequency: Preplacement and age determined.
(2) Additional visual requirements (See section 3.2.5):
(a) Binocular vision.
(b) Normal color discrimination (if colored signals are essential to operations).
(c) Bifocal correction lenses are authorized.
(3) Hearing: Hearing loss with or without aid, averaged in the frequencies of 500, 1000, and 2000 hertz (Hz) of no greater than 30 decibel (dB) in better ear.
(4) If a question of cardiovascular fitness arises, evaluation by the employee's private medical doctor, and the appropriate documentation, may be needed.
(5) Strength, endurance, agility, coordination, dexterity, and reaction speed consistent with normal, healthy physiology, and assigned task.
(6) No history of seizures, emotional instability, or physical conditions or defects which could render the employee ineffective or a hazard to themselves, others, or the equipment being operated.
d. Crane Operator/High
(1) This section also includes Cab/Pulpit Operated Cranes.
(2) Frequency: Preplacement and age determined.
(3) Additional visual requirements (See section 3.2.5):
(a) Binocular vision.
(b) Normal color discrimination (if colored signals are essential to operations).
(c) Bifocal correcting lenses are authorized, but larger near vision corrective segments are recommended.
(4) Hearing: Hearing loss, with or without aid, averaged in the frequencies of 500, 1000, and 2000 Hz of no greater than 30 db in better ear.
(5) Ability to complete 95 percent Predicted Age Adjusted Maximal Heart Rate (PAAMHR) of the Bruce Protocol* for treadmill exercise stress test without evidencing significant physiologic, electrocardiographic, or clinical pathology.
(6) Strength, endurance, agility, coordination, dexterity, and reaction speed consistent with normal, healthy physiology for assigned task, ability to climb a vertical ladder.
(7) No history of seizures, emotional instability or physical conditions or defects which could render the employee ineffective or a hazard to themselves, others, or the equipment being operated.
e. Diver
(1) Frequency:
(a) Open water. Preplacement and age determined. (There may be frequency requirements that are unique to a requesting agency.) See Section f. (Down Range/Shipboard, and Remote Assignment).
(b) Nonopen water. NASA Standard 8719.10, Standard for Underwater Facility and Non-Open Water Operations.
(2) The following is included in the physical
examination:
(a) A complete history is obtained from the employee.
(b) Routine laboratory and CXR.
(c) The examination by the physician includes a review of the history with elaboration of any positive findings, and further examination of the HEENT to rule out obvious anatomic problems, sinus difficulties, or dental disease.
(d) Some employers may require long bone x-rays on initial examination. X-rays are performed on subsequent examinations only at the examining physician's request.
(e) The employee completes 95 percent PAAMHR on the Bruce Protocol* without clinical and/or ECG signs/symptoms of significant cardiac disease.
(f) A positive statement regarding ability to perform the Valsalva maneuver is noted.
(g) The cardiovascular, respiratory, neuromuscular and orthopedic examination assure no gross abnormalities in those systems.
(h) If there is reason to hold the medical qualification until additional medical evaluation is obtained, then inform the employee and NASA that the employee is not to dive until cleared by the OHP physician.
(i) Examination for underwater facility and
non-Open Water operations require an examination by a certified (Hyperbaric or underwater) physician.
*These are KSC requirements recommended Agencywide for Open Water divers.
f. Down Range/Shipboard and Remote Assignments
(1) The employee must be medically qualified for assigned job and have completed a pretravel questionnaire for travel clearance.
(2) Frequency: Preassignment and age determined by the following directive: Downrange and Remote Assignment. The following schedule applies for Eastern Test Range Shipboard: *
|
Category/Age
|
Exam Cycle
|
|
17-24 years
|
Every 5 years
|
|
25-49 years
|
Every 3 years
|
|
50-59 years
|
Every 2 years
|
|
60 years and up
|
Annually
|
|
Masters
|
Annually
|
|
Chief Mates
|
Annually
|
|
Chief Engineers
|
Annually
|
|
1st Asst. Engineer
|
Annually
|
(3) Immunizations:
(a) Immunizations may be required for all employees traveling outside the continental United States on official business. All
required immunizations are administered prior to departure. The OHP Medical Director confirms immunization requirements when appropriate.
(b) Before administering immunizations to female employees, check their "pregnancy" status and determine what immunizations their physician has approved. The OHP physician may recommend a waiver of the immunization on the immunization record if clinically indicated.
(c) Refer to the CDC "Blue Sheet" (weekly summary of countries with infected areas of Quarantined Disease and Health Information for International Travel) for current information on immunization requirements, access on Internet at http://www.cdc.gov. Immunization and country information can be obtained by contacting International SOS at 1-800-523-8930.
(d) The following routine immunizations are recommended for travel to or on all range bases, sites, ships, outside the continental United States:
(i) Measles, Mumps and Rubella (MRMR).
(ii) Tetanus/Diphtheria every 10 years.
(iii) Polio with one booster.
(iv) Yellow Fever every 10 years.
(v) Hepatitis B series. (Accompanied by the
appropriate bloodborne pathogen training. This vaccination series may be reserved only for health care workers, those assigned to first aid duties, or employees that are planning on extended tours of duty in areas where Hepatitis B is endemic.)
(vi) Hepatitis A series.
(4) Tuberculosis (TB) skin test status [Mantoux, Purified Protein Derivative (PPD) ] is documented prior to travel. If the PPD is positive (abnormal), then a physician examination and CXR are performed. If the employee has a documented history of a positive PPD,
then the physician determines if further testing is needed prior to clearance for international travel. If the CXR is abnormal and suggestive of active tuberculosis, then a consultation occurs to determine additional testing and treatment as appropriate. This information is documented in the record before travel clearance is approved. Post-travel followup for travelers with negative PPD is recommended to the employee. Refer to Section 3.4.5, Tuberculosis Prevention and Skin Testing, for more detailed information.
(5) A records release form is inserted in each interim folder on SHIPSDUTY physical examinations. The OHP medical personnel obtain the employee's signature on the release form.
(6) All female mariners are eligible for an annual Pap test, pelvic examination, and manual breast examination. Screening mammography according to ACS or American College of Obstetricians and Gynecologists recommendations is encouraged.
(7) All remote assignment physical examinations include an initial blood type. Employees are informed of the blood type, and it is noted in the medical record.
(8) All employees returning from a remote assignment with significant illness (hospitalization or early return due to illness) are disqualified for future remote assignment until approved by appropriate authority.*
(9) Insulin-dependent diabetes mellitus is disqualifying for remote assignment.*
(10) Noninsulin-dependent diabetes mellitus may be disqualifying for remote assignment, depending on the adequacy of treatment, side effects, end organ effects, and current health status of the employee.*
(11) History of myocardial infarction, coronary artery bypass graft or percutaneous transthoracic coronary angioplasty is disqualifying for remote assignment.*
NOTE: There are no waivers for the African transatlantic abort sites.*
g. Federal Aviation Administration Personnel
(1) Nonjob-related FAA physical examinations on NASA and contractor employees are performed at the discretion of the local contracting officer. FAA examinations that are job related or requested by the contracting office are performed for NASA and contractor employees.
(2) Frequency is as required by FAA regulations. (See the FAA Guide for AME for applicable standards and examination procedures).
(3) Only those test results required for the specific class of FAA requested physical are transposed to the FAA Form 8500-8. The following item notes apply:
(a) Items 1 - 24: Medical Services personnel ensure applicant has completed FAA Form 8500-8. All blocks are completed. AME reviews for history, other relevant data, or omissions.
(b) Items 25 - 48: Completed by AME. Review and apply the applicable chapter of AME Guide when abnormalities are reported.
(c) Items 49 - 59: All elements completed by medical services personnel.
h. Firefighter
(1) Frequency: Preplacement and age determined.
(2) All incumbent firefighters, firefighters/drivers, crew chiefs, and assistant crew chiefs receive complete physical examinations and medical certification. The following special requirements apply:
(a) Audiological hearing aids are not permitted.
(b) Psychologically, employee must possess mental and emotional stability. Any condition, which would cause applicants to be a hazard to themselves or others, is cause for disqu
alification.
(c) Graded Exercise Test (GXT): Diagnostic symptom limited GXT (95 percent PAAMHR) at preplacement and on periodic physical examinations.*
(d) Vision: Distant vision, uncorrected, tests at least 20/70 (Snellen) in one eye and 20/100 (Snellen) in the other eye, and corrected to at leas 20/20 in one eye and 20/40 in the other eye. Near vision corrected to 20/40 in both eyes.
i.
Food
Handler
Use guidelines established for Primary Crew Contact described in section 3.2.5.
j. Fuel Handler/Contingency Crew
(1) Frequency: Complete preplacement with annual laboratory screening [blood (CBC), renal (U/A), liver (SMAC)].
(2) Employees must have the appropriate certifications to perform the basic job to which they are assigned. That is, their limitation or restriction permits them to function in the assigned position, e.g., employees with prosthetic arms would be excluded
from work on scaffolding.
(3) Employees should be free of liver, renal, or significant cardiac or pulmonary disease that may be adversely affected by exposure to toxic propellants. Judgment of the OHP physician prevails, based, on considerations of examination, laboratory, and ancillary data.
*KSC requirement recommended Agencywide.
k. Hazardous Materials Emergency Response Team
(1) Reference: OSHA 29 CFR Part 1910.120 and American National Standards Institute (ANSI) "Standard on Medical Requirements for Firefighters," National Fire Protection Association upgraded 1994. The response to HAZMAT usually comes from within the fire services and responds to actual or potential leaks or spills of hazardous substances. Members meet the requirements for Firefighter (See section 3.2.4.2.) and SCAPE (See section 3.2.4.2.) in addition to these special frequency examinations. (Hazardous waste workers, other than the HAZMAT emergency response team, meet the requirements for hazardous waste workers - see section 3.2.3.2)
(2) Frequency: A complete physical at preplacement; every 2 years to age 50, then annually; at termination of HAZMAT exposure (if no physical examination performed within the previous six months),
and ad hoc for exposure occurrence. Annual surveillance examinations may be required if exposures dictate (See Section 3.2.3, Surveillance Examinations).
l. Heavy Ordnance
(1) This section also includes explosives handler.
(2) Frequency: Preplacement and age determined (except military personnel and military contractors at Cape Canaveral Air Force Station, which are annually and at termination.)**
(3) A history of seizures, emotional instability or physical conditions or defects which could render employees ineffective or a hazard to themselves, others, or the materials and equipment being handled or operated is considered disqualifying.
m. High Crew/Spider
(1) Frequency: Preplacement and age determined.
(2) Special attention is directed to exclude any organic or psychological problems contraindicating work on high structures, or any organic problems contraindicating specific duty that the employee is required to perform on a high crew duty, e.g., painting, sandblasting, general mechanic duty.
(3) Subject should be free of psychoemotional instability.
n. Locomotive Engineer/Crawler-Transport
(1) Frequency: Preplacement and age determined.
(2) Employees should be free of electrocardiographic and physiologic signs/symptoms of significant heart disease and complete a 95 percent PAAMHR GXT, using the Standard Bruce Protocol.*
* KSC requirement recommended Agencywide.
** KSC unique.
o. Motive (Heavy) Equipment Operators
(1) Any vehicle over one ton is considered to be heavy equipment.
(2) Frequency: Preplacement and age determined.
(3) There should be no significant medical problems that might lead to sudden incapacitation, such as seizure history, cardiovascular disease or, diabetes mellitus.
(4) There should be no history of emotional instability or physical conditions or defects, which could render employees ineffective or a hazard to themselves, others, or the equipment being used.
p. Multiple Passenger Vehicle Operator
(1) Reference: 49 CFR Part 391.41.
(2) Frequency: Preplacement and age determined to comply with DOT Commercial Drivers License requirements.
(3) Examination includes a GXT. The employee must achieve as a minimum 95 percent of their PAAMHR without exhibiting any electrocardiographic or physiologic evidence of significant cardiovascular disease.*
*KSC requirement recommended Agencywide.
q. Vehicle Certification
(1) Reference: 49 CFR Part 391.41.
(2) Frequency: Every 2 years (biannually).
(3) DOT physical examination standards apply. Employees may be physically qualified if they--
(a) Have no loss of a foot, a leg, a hand, or an arm, or have been granted a waiver.
(b) Have no impairment of
1) a hand or finger which interferes with prehension or grasping; or
2) an arm, foot, or leg which interferes with
their ability to perform normal tasks associated with operating a motor
vehicle; or
3) any other significant limb defect or limitation which interferes with their ability to perform normal tasks associated with operating a motor vehicle; or
4) have been granted a waiver.
(c) Have no established medical history or clinical diagnosis of diabetes mellitus currently requiring insulin for control.
(d) Have no current clinical diagnosis of myocardial
infarction, angina pectoris, coronary insufficiency, thrombosis, or any
other cardiovascular disease of a variety known to be accompanied by syncope, dyspnea, collapse, or congestive heart failure.
(e) Have no established medical history or clinical
diagnosis of a respiratory dysfunction likely to interfere with their ability to control and drive a motor vehicle safely.
(f) Have no current clinical diagnosis of high blood
pressure likely to interfere with their ability to operate a motor vehicle
safely.
(g) Have no established medical history or clinical
diagnosis of rheumatic, arthritic, orthopedic, muscular, neuromuscular, or
vascular disease, which interferes with their ability to control and operate a motor vehicle safely.
(h) Have no established medical history or clinical
diagnosis of epilepsy or any other condition, which is likely to cause loss of consciousness or any loss of ability to control a motor vehicle safely.
(i) Have no mental, nervous, organic, or functional disease or psychiatric disorder likely to interfere with their ability to drive a motor vehicle safely.
(j) Have distant visual acuity of at least 20/40 in each eye without corrective lenses or visual acuity separately corrected to 20/40 or better with corrective lenses. Field of vision of at least 70 degrees in the horizontal meridian in each eye, and the ability to recognize the colors of traffic signals and devices showing standard red, green, and amber. Monocular employees are not qualified to operate commercial motor vehicles.
(k) Can perceive a forced whispered voice in the better ear at not less than five feet with or without the use of a hearing aid or if tested by use of an audiometric device.
(l) Do not have an average hearing loss in the better ear greater than 40 dB at 500 Hz, 1,000 Hz, and 2,000 Hz with or without a hearing aid when the audiometric device is calibrated to ANSI standards.
(m) Do not use Schedule I drugs, an amphetamine, narcotic, or any other habit-forming drug. The exception would be that employees might use such a substance or drug if an OHP physician who is familiar with their medical history and assigned duties prescribes it. In addition, the OHP physician advised the employees that the prescribed substance or drug would not adversely affect their ability to safely
operate a motor vehicle.
(n) Have no current clinical diagnosis of alcoholism.
(4) Employees who are deemed not medically qualified to operate motor vehicles according to the above criteria are considered for a waiver only in the following circumstances:
(a) Loss of a foot, leg, hand, or arm with little residual impairment of the employee's ability to safely operate motor vehicles.
(b) Impairment of a hand or finger that interferes with grasping, if the impairment does not impair the employee's ability to safely operate motor vehicles.
(c) Impairment of an arm, foot, or leg that does not
interfere with the employee's ability to safely operate motor vehicles.
(d) Any other significant limb defect or limitation, which does not significantly interfere with the employee's ability to safely operate motor vehicles.
(5) Requests for DOT waivers are submitted by the employee and the employer to the Regional Director, Federal Motor Carrier Safety.
r. Noncrew Flying
(1) Preassignment and age determined.
(2) This procedure is followed for physical examinations on NASA and contractor employees who are required to fly on NASA aircraft as noncrew members in performance of their jobs. These include fire, security, safety, photo-optics, and other observer
personnel.
(3) The scope of the physical examination is the same as that required for FAA Class III. The results are recorded in the medical record or on an approved form. The FAA Forms are not to be used.
(4) The records of employees who do not meet the standards for FAA Class III examination in accordance with the FAA manual are referred to the OHP Medical Director for review. An entry in the medical record is made by the examining physician to state the reason(s) for disqualification. This note in the medical record will also cite the reference for disqualification from the FAA Guide for AME.
(5) The OHP Medical Director may determine that the reason for the disqualification would not threaten the well being of the employee or the mission and grant a waiver to perform the assigned duties.
s. Selfcontained Atmospheric Protective Ensemble (SCAPE)
(1) This section also includes the Self Contained Breathing Apparatus and the Liquid Air Pack.
(2) Reference: OSHA 29 CFR Part 1910.134 (The OSHA Respiration Protection Standard is applicable to two physical examination categories):
(a) SCAPE, which addresses the requirements for employees assigned to enter atmospheres that are immediately threatening to life and health or are expected to perform rescue/evacuation operations.
(b) Other employees that are assigned to jobs that necessitate the wearing of respiratory protection other than SCAPE [See section 3.2.5].
(3) Frequency: Preplacement and age determined (for employees assigned to enter atmospheres that are immediately threatening to life and health or are expected to perform rescue/evacuation operations).
(4) General considerations:
(a) It is recognized that the SCAPE suit, although a protective garment, can, under certain circumstances, present a significant hazard to employees who are not in good physical condition or who have some medical or physical limitations.
(b) An inherent requirement is for employees to be physically able to evacuate an area quickly while in the SCAPE suit. Evacuation might entail rapid ascent or descent of stairs or a ladder or even to run some distance and might require that the employee assist another adult in rapid egress.
(c) The first or basic requirement for SCAPE suits is that employees have no gross physical defect that would prevent normal mobility, performance of job, ability to evacuate an area rapidly, or to assist another to the point of supporting a major part of another adult's weight.
(d) The second general concept is that employees are able to see well enough with correction to perform their usual tasks and to be able to see well enough without correction to evacuate a position under adverse circumstances such as relative darkness.
(e) The third basic principle is that employees have no chronic or active disease process that has a reasonable probability of resulting in an emergency evacuation of employees or their partners in the buddy system, or of adversely impacting the integrity of the operation.
(f) A fourth principle applies to employees who are theoretically in the high risk group for acute cardiopulmonary and vascular emergencies who might not have an otherwise disqualifying condition. For this last consideration, the determinant is the judgment of the examining physician, after performing complete physical examinations with special cardiovascular testing for SCAPE suit duties.
(5) Specific requirements for SCAPE duty qualifications:
(a) GXT is performed at each examination (Completes stage III of Bruce Protocol without significant electrocardiographic or physiological abnormalities).*
(b) Acceptable visual acuity, as outlined in the section 3.2.5.)
(c) Normal depth perception is not required unless it is a specific requirement of the employee's duty while in SCAPE suit, e.g., crane operator.
t. Security Personnel
(1) Frequency: Complete physical preplacement and age determined.
(2) Visual standards apply as listed in section 3.2.5.
(3) GXT: Completes 95 percent PAAMHR on the Bruce Protocol without clinical and/or ECG signs/symptoms of significant cardiac disease. Consideration is given to those employees who are on beta blockers in regards to maximum heart rates achieved on the GXT.*
(4) The OHP physician must recognize that other security duties, such as Special Weapons And Tactics (SWAT) team duties, require more stringent physical activities and may not be appropriate for certain employees. Such factors must be considered when certifying
security personnel.
*KSC requirement recommended Agencywide.
u. Solid Rocket Booster Retrieval
(1) Frequency: Complete physical, preplacement and age determined
(2) Visual standards apply as listed in. section 3.2.5.
(3) Certifications for other duties (section 3.2.4.2 Diver, Down Range) are current.
3.2.5 Special Purpose Examinations
3.2.5.1. Introduction
Centers of other agencies/contractors authorized to use services provided by the OHP facilities may request examinations that are not included in any of the other categories. Examples of these types of examinations are disability, retirement, termination, Fitness For Duty (FFD), Return To Work (RTW), independent medical examination, and primary crew contact.
3.2.5.2 Process Description
3.2.5.3 Employment Situations
a. Fitness for Duty - General
FFD examinations are usually performed when employees are returning to work after an illness or injury. These examinations may also be requested when a change in the employee's health or performance is observed or suspected. Determination of fitness requires identification and documentation of the physical requirements and essential functions for the job. Firsthand familiarity with the job by the OHP physician is invaluable.
b. Fitness For Duty - Civil Service
(1) The office requesting the FFD examination indicates on Standard Form (SF) 88 the functional and environmental factors required for the employee to meet the standards for the job classification.
(2) The office requesting the FFD examination obtains the employee's consent to have an FFD physical prior to the examination.
(3) If possible, the examination is completed in 1 day.
(4) A cover letter from the requesting office accompanies the SF 88 with instructions for completion and includes the forwarding procedure for the completed SF 88.
c. Fitness For Duty - Contractor
FFD examinations are accepted when the requesting employer or employee meets the following criteria:
(1) A job description that specifies physical performance requirements accompanies the request.
(2) The proposed employee has been previously examined under and has met the requirements of the published Physical Examination Standard applicable to the job for which fitness is questioned.
(3) A supervisory statement accompanies the request that describes how the employee does not measure up to the requirements of the job.
(4) The employer requires the employee to obtain clearance for RTW from OHP medical personnel after an illness/injury and that this clearance is recorded in the employee's medical records retained by OHP medical personnel.
(5) Requests for FFD examinations not meeting all stated criteria are returned to the originator without action.
d. Return To Work
(1) RTW physicals are requested by authorized users of the OHP medical services. The procedures are as follows:
(a) The OHP Medical Director receives a "Letter of Instruction" and two copies of SF 88 from the office requesting the physical.
(b) The physical is scheduled, and the requesting office is notified of the date and time.
(c) The employee has an interview with an OHP physician, who ascertains the required extent of the evaluation that is needed.
(d) After the employee's examination, the OHP physician fills out the duplicate copy of the SF 88 and forwards it to the requesting office.
(2) Most other RTW physicals do not require a full physical examination. OHP medical personnel determine the extent of the examination and appropriate laboratory testing. If fasting laboratory work is needed, the employee is scheduled for a followup examination as required. RTW certification and statement of limitations (and duration), if any, are documented along with recommendations for disposition. These are distributed as follows:
(a) Original given to employee to give to his supervisor.
(b) Copy to Company Safety Officer, for occupationally related issues.
(c) Copy filed in employee's medical record.
3.2.5.4 Visual Examinations
a. General Examinations
(1) Two types of functional eye examinations are given for job certifications during partial or complete physical examinations: visual acuity and color perception. Administration of these tests is suggested for such positions as Quality Control Inspectors, Electricians, Welders, Solderers, Crane Operators, or any other personnel who may be required to have specified visual acuity and distinguish color vision as part of performing their normal job duties. Depth perception and visual fields may also be required.
(2) Job classifications that require Federal Aviation Administration (FAA) and Department of Transportation (DOT) certifications have differing and additional requirements. It is recommended that FAA and DOT regulations be consulted for actual requirements.
b. Specific Recommendations
(1) For all complete physicals record at least the visual acuity and color perception.
(2) Visual acuity should be evaluated with tests such as Snellen (far) and Jaeger (near).
(3) Color perception should be evaluated with tests such as Dvorine Plates or Ishihara Plates.
(4) Frequency: Annual examinations are suggested, but individual Centers may establish more frequent requirements.
3.2.5.5. Work Place Exposures
a. Bloodborne Pathogens
(1) Reference: 29 CFR Part 1910.1030 (more detailed information follows in Section 3.4, Infection Control).
(2) Frequency: For BBP, initial Hepatitis-B vaccine is offered to all employees identified by a company's Exposure Control Plan (as required by Federal Register 29 CFR Part 1910.1030). Annual training is also required by the applicable CFR.
(3) If employees decline the vaccine, they sign a mandatory declination statement. If at a future time an employee chooses vaccination, then a resubmission request for vaccination is honored.
(4) A copy of the declination statement for those who decline vaccination for Hepatitis B is maintained. Access to the record for inclusion in the Exposure Control Plan documents is permissible.
(5) All employees who are occupationally exposed to BBP's are evaluated according to the current CDC guidelines.
b. Laser Workers
(1) References: AFOSH Std. l6l-l0 dated 5/30/80, "Health Hazards Control for Laser Radiation", ANSI Z136.1 - 1986 "Safe Use of Laser."
(2) Frequency: As determined by risk (see below).
(3) The employer places all employees whose duties require their routine presence within the minimal safe distance of a laser/laser system (as defined by the Radiation Protection Officer) in a medical surveillance program. The extent of the surveillance program is determined in part by the following:
(a) The risk classification of the employees as assigned by the Radiation Protection Officer.
(b) The wavelengths of radiation being utilized. These parameters are specified to the medical facility by the requesting organization so that the appropriate minimum test requirements may be selected. This does not include visitors who are adequately protected by appropriate protective eyewear or other administrative/procedural controls.
(4) Laser worker risk classification categories are as follows:
(a) Incidental Personnel - Exposure possible but unlikely that they will be exposed to laser energy sufficient (Class IIIb and IV) to cause physical damage, e.g., custodial, clerical, supervisory personnel.
(b) Laser Workers - Personnel who work routinely in laser environments with higher powered lasers (i.e.,Class IIIb and up) and are ordinarily protected by engineering/procedural type controls.
(5) Medical surveillance for incidental personnel includes baseline visual acuity. Laser workers receive ocular and medical histories, visual acuity, Amsler Grid Test, and color vision tests as described below.
(a) Ocular History. The past eye history and family history is reviewed. Any current complaints related to the eyes are noted. Inquiry is made into the general health tatus with a special emphasis upon systemic diseases that may produce ocular problems. Use of corrective lenses is recorded. Certain medical conditions may cause the laser worker to be at an increased risk for chronic exposure. Use of photosensitizing
medications, such as phenothiazines and psoralens, may lower the threshold for biological effects in the skin, cornea, lens and retina. Aphakic individuals would be subject to additional retinal exposure from blue light and near ultraviolet and ultraviolet laser radiation. Unless chronic viewing of these wavelengths is required, there should be no reason to deny laser operations to these aphakic individuals.
(b) Visual Acuity. Visual acuity for far and near vision is measured according to established procedure. Refraction corrections should be made if required for both distant and near test targets. If visual acuity does not correct to 20/20 for distance and Jaeger 1 for near, an examination is required as listed in section 3.2.5.
(c) Macular Function. An Amsler grid or similar pattern is used to test macular function for distortions and scotomas. If any distortions or missing portions of the grid pattern are present, the test is not normal.
(d) Color Vision. Color vision discrimination is documented by Ishihara or similar color vision tests.
(e) Examination of the Ocular Fundus with an Ophthalmoscope. This portion of the examination is administered to
individuals whose ocular function tests, which are described above, are
not normal. The points to be covered include the presence or absence of
opacities in the media; the sharpness of outline of the optic disc; the color of the optic disc; the depth of the physiological cup, if present; the ratio of the size of the retinal veins to that of the retinal arteries; the presence or absence of a well-defined macula and the presence or absence of a foveal reflex; and any retinal pathology that can be seen with an ophthalmoscope (hyperpigmentation, depigmentation, retinal degeneration, exudate, as well as any induced pathology associated with changes in macular function). Small deviations from normal should be described and carefully localized. Dilation of the pupil is required.
(f) Skin Examination. Examination of the skin is not required for preplacement examinations of laser workers; however, it is suggested for employees with history of photosensitivity or working with ultraviolet lasers. Any previous dermatological abnormalities and family history is reviewed. Any current complaints concerned with the skin are
noted as well as the history of medication usage, particularly concentrating on those drugs which are potentially photosensitizing.
(g) Other Examinations. Further examinations are performed as deemed necessary by the OHP physician.
(6) Medical Referral Following Suspected or Known Laser Injury:
Any employee with a suspected laser eye and/or skin injury should contact the Occupational Health facility. The postexposure examination includes items as listed above as deemed necessary by the OHP physician.
(7) Records and Record Retention:
Complete and accurate records of all laser medical examinations are maintained for all personnel included in the medical surveillance program. Records are retained for at least 30 years. The
results of medical surveillance examinations are discussed with the employee.
c. Primary Animal Contact
(1) All requests for primary animal contact are
, approved by the NASA Center Contracting Officer's Technical Representative's Office.
(2) Classification:
(a) Access
(b) Direct
(c) Primate
(3) Frequency: Annually
(4) A complete history is recorded.
(5) Laboratory
, procedures:
All require SMAC, CBC, U/A, C-reactive protein, Rapid Plasma Ragin (RPR) and stool for bacterial examination for Salmonella and Shigella. (Frequency of stool specimen: Initial and at the discretion of the OHP physician).
(6) Immunizations: Tetanus/Diptheria every 10 years or sooner if clinically indicated and other vaccines as indicated.
(7) PPD: Not more frequently than annually. The PPD is not performed on employees with a known/documented, previously positive test. If PPD is abnormal, a followup CXR is performed to survey for possible TB disease. Followup CXR in subsequent years is at the discretion of the OHP physician. (See section 3.2.4.2, Immunizations).
(8) Examination by an OHP physician to include checking the employee's hands, face, neck, scalp, nose, throat, and feet to determine absence or presence of infectious disease.
d. Primary Crew Contact
(1) Standards are established in the Health Stabilization Program administered at Johnson Space Center (JSC).
(2) Frequency: Crew food handlers and crew quarter
custodians every six months. All others annually. Expiration of the
certification is the last day of the month in which the physical examination was completed, plus six or 12 months, whichever applies.
(3) Use JSC Forms 115A, 115B, 115C
(4) Laboratory procedures:
(a) RPR, Serum Glutamic-Oxaloacetic Transaminase (SGOT), CBC with differential, U/A.
(b) Additional laboratory requirements for Food Handlers:
(c) Stool culture for Salmonella, Shigella, and examination of the stool for ova and parasites.
(d)Throat culture for significant respiratory pathogens such as Beta-hemolytic and Streptococci.
(5) Immunization requirements:
(a) Tetanus/Diptheria every 10 years.
(b) MMR if no history of immunization or negative serologies.
(c) Influenza and Hepatitis A vaccination is offered but not required.
(d) PPD: Annual PPD screening required. The PPD is used except for those with a history of a positive PPD. The OHP physician reviews all positive PPD tests. CXR is indicated for all positive PPD's. With a history of a positive PPD, annual CXR examinations are at the discretion of the OHP physician.
(6) All employees with this certification view the Primary Crew Video.
(7) Temporary Disqualification: Whenever an employee
on the Health Stabilization Program is temporarily disqualified/decertified due to illness, their Primary Crew Contact badge is held by OHP medical personnel. When the employee is cleared to return to full duty, the badge is returned to the employee.
e. Ionizing Radiation Workers
(1) Frequency: Stated below, depending upon examination category, the radiation medical examination includes, but is not limited to, a careful history, complete physical examination, CXR, CBC, U/A, SMAC and other bioassays as indicated by the OHP physician. If a physical examination has been conducted within the previous six months and has been duly recorded in the employee's health record, it may, at the discretion of the OHP physician, be accepted in whole or in part in lieu of the corresponding sections of the radiation medical examination. Complete examinations conducted more than 6 months previously, may be utilized with appropriate supporting information and a signed interval note by the cognizant OHP physician in line 73 of the SF 88 (or equivalent). A physical examination conducted for one purpose is valid for any other purpose within the prescribed validity period if that physical contains the proper data. If the examination is deficient in scope, only those tests and procedures needed to meet the additional requirements are performed.
The results are recorded, and the OHP physician signs the appropriate approval.
(2) Types of radiation medical examination are as follows:
(a) Preplacement examination: All employees who are being considered for assignment to duties or occupations requiring exposure to ionizing radiation or the handling of radioactive material are given a medical examination prior to assignment or transfer to those duties or occupations. This examination is performed to ensure that a rospective employee is physically qualified for occupational exposure to ionizing radiation. People who are not routinely exposed to ionizing radiation during their normal occupations and who are not likely to exceed 0.5 roentgen-equivalent-man (rem) per year or 0.125 rem per quarter are not
required to have preplacement examinations. These people include visitors,
messengers, servicemen, delivery men, and certain crewmembers or employees
whose exposure is truly sporadic.
(b) Re-examination: Employees who are exposed to ionizing radiation in the course of usual duty or employment are examined every 3 years. These examinations are required to ensure that employees receiving occupational exposures above the limits permitted by the general population continue to meet the physical standards. Employees who receive less than 0.5 rem each year are exempt from reexamination.
(c) Situational examination: A special medical examination is given as soon as it can be scheduled for any employee who has exceeded the current radiation protection standards for occupational exposure, or has possibly ingested or inhaled a significant amount of radioactive material, or as deemed necessary by the OHP physician.
(d) Termination examination: All employees who have
received greater than 0.5 rem in any 1 calendar year are given a radiation
physical examination at the termination of their employment. These
examinations are required in order to verify the physical status of employees at the end of their employment. Upon transfer from duties as radiation workers, an entry is made on SF 88 (or equivalent) that reflects the following statement: "Termination Radiation Physical Examination required prior to release or retirement".
(3) Medical history:
For the preplacement examination, a complete medical history is obtained. The medical histories on all employees receiving radiation medical examinations specifically include the following:
(a) History is completed on each employee.
(b) Work history to determine the amount of ionizing
radiation previously received as a result of occupational exposure.
(c) Past history to evaluate any previous malignancies or pre-malignant lesions.
(d) History of radiation therapy that may have been
previously received.
(e) Family history of malignancies, pre-malignant lesions, infertility, cataracts, and congenital or familial defects.
(f) For reexaminations, the medical history may be limited to that interval of time since the last radiation physical examination. The OHP physician is alert for symptoms of chronic illness and anxiety regarding exposure to radiation.
(4) For situational examinations, the medical history may be limited to a detailed account of the employee's activities at the time the exposure occurred and to an interval history since the last radiation physical examination. Evidence of malignant and pre-malignant lesions, lenticular opacities, and other conditions that could be related to radiation is elicited. Positive entry in the documentation record describes ophthalmoscopic examination, e.g., "media clear" or "no lenticular opacities", or if no clear findings are apparent.
(5) If lenticular opacities are identified, slit lamp
examinations are performed. All employees who are 36 years of age or older
shall have a slit lamp examination. Slit lamp examinations are conducted by an ophthalmologist and properly documented.
(6) Lenticular opacities in the following categories are submitted to the OHP Medical Director for review: senile, traumatic, or metabolic, in a posterior subcapsular location. Opacities in the following categories need not be submitted for review (unless in a posterior subcapsular location): punctate pigment, vacuole, Y suture, hyaloidea
artery remnant, and Mittendorf spot.
(7) Laboratory Procedure are as follows:
(a) CXR required on all preplacement examinations
(b) CBC with differential, U/A, and SMAC.
f. Respirator, Occupational, (Non-SCAPE)
(1) Reference: 29 CFR 1910.134 OSHA Respiratory Protection Program Standard [See section 3.2.4, s., Self Contained Atmospheric Protective Ensemble (SCAPE), for more information.]
(2) Frequency: Initial medical evaluation and subsequent examinations depend upon the type of respirator and conditions under which a respirator is used, as well as the medical conditions/problems that are identified during the medical evaluation. All respiratory users in this category have at a minimum a medical questionnaire update and medical evaluation every 5 years. Employees with identified medical problems that can be assumed not to be static conditions, may be reevaluated on a more frequent schedule. The certifying examiner or OHP physician determines the frequency.
(3) General considerations are as follows:
(a) Respiratory protection equipment is worn to protect employees from a hazardous environment. The character of the hazardous environment and the above medical evaluation frequencies determine the frequency and extent of the medical evaluation. The specifics of the type of respirator used, the environment under which those respirators are used, and the frequency of use is all covered in the Respirator Medical Evaluation Questionnaire.
(b) In addition to the hazardous environment in which
employees may work, respiratory protection equipment itself may produce an
abnormal physiologic demand upon the body, and add to the psychological and
physical stresses that exist. Devices such as SCAPE suits add considerable
weight and increased physiologic demands on the cardiovascular, pulmonary, and musculoskeletal systems. Positive pressure demand systems complicate the normal physiology of breathing and add additional pulmonary, cardiovascular, and physiological stresses. The respiratory protective gear or exposure to environmental hazards may aggravate certain existing abnormalities. All these factors are considered in the physical and psychological screening examinations.
(c) The screening physical evaluation is accomplished prior to training and assignment to a respiratory protection position. It identifies medical and psychological problems that could jeopardize the welfare of the employee, coworkers, or the operation to which the employee is assigned.
(4) Special requirements for Respiratory Medical Certification:
A medical and work history to identify medical conditions that may adversely affect the employee's ability to safely use a respirator is covered with the use of the Respirator Medical Evaluation Questionnaire. An additional medical evaluation is required for any positive answers to questions on the questionnaire. This medical evaluation is of such degree that it provides enough additional information to aid the OHP physician in making a determination of whether the employee can safely wear and operate with the requested respirators.
(5) The Respirator, Occupational, Non-SCAPE certification includes wearing the following respirators: Half Face Air Purifying, Full Mask Air Purifying, Half Face Partial Air Purifying Respirator (PAPR), Supplied Air Respirator (SAR), Full Face PAPR, SAR Helmet/Hood, SAR Half Face Continuous Flow, SAR Full Face Continuous Flow and SAR Positive Pressure.
(6) Employees working in these devices need to demonstrate general good health. Any positive responses on the questionnaire that indicate problems with cough, phlegm, asthma, episodic wheezing, diabetes, seizures, or claustrophobia need to have OHP physician evaluation. Cardiovascular problems need to be well defined and determined to be under adequate control, without acute problems such as congestive heart failure or angina.
(7) No allowed waivers for respirator usage are expected. OHP physicians' concerns/questions about specific respirator use can result in field testing of the respirator, permitting additional information for the OHP physician to make a final determination of medical certification. Field testing normally is used only on rare occasions.
g. Respirator, Other
(1) Reference: 29 CFR 1910.134, OSHA Respiratory
Protection Program Standard.
(2) [See section 3.2.4, s, Self-Contained Atmospheric Protective Ensemble (SCAPE) for more information.] This category of examination applies to employees who are not assigned regularly to work in any environment presenting a respiratory hazard. They may be advised to wear a dust mask or filtering face piece as a personal protection on a temporary basis. They may need training and appropriate certification for these tasks, and hence medical clearance.
(3) NOTE: OSHA has excluded Emergency Life Support
Apparatus respirators from the medical requirements in the 29 CFR 1910.134 standard.
(4) Frequency: Initial medical evaluation and subsequent examinations at a frequency, depending upon the medical conditions/problems that are identified during the initial evaluation. All respiratory users in this category have at a minimum a medical questionnaire update and medical evaluation every 10 years. Employees with identified medical problems, which can be assumed not to be static conditions, may be reevaluated on a more frequent schedule. The certifying examiner or OHP physician determines the frequency.
(5) General considerations are as follows:
(a) Respiratory protection is worn to protect the worker from a hazardous environment. The types of respirators and conditions for use are identified in the Respirator Medical Evaluation Questionnaire.
(b) The screening physical evaluation is accomplished prior to training and assignment to a respiratory protection position. The evaluation identifies medical and psychological problems that could jeopardize the welfare of the employee, coworkers, or the operation to which the employee is assigned.
(6) Special requirements for respiratory medical
certification:
A medical and work history to identify medical conditions that may adversely affect the employee's ability to safely use a respirator are covered with the use of the Respirator Medical Evaluation Questionnaire. An additional medical evaluation is required for any
positive answers to the questions on the questionnaire. The medical evaluation is of such degree that it provides enough additional information to aid the OHP physician making a determination of whether the employee can safely wear and perform required functions using the requested respirator.
(7) Respirator users in this category need to be in
generally fair condition with no,significant limiting physical factors that would duly limit their or other employees ability to perform a proper egress.
h. Tuberculosis Control
(1) Frequency: Annually in health care workers. Biannually in daycare workers.
(2) Program is limited to administration, documentation and interpretation of TB skin testing and appropriate followup for positive tests (or a history of a positive skin test).
(3) Employees with a history of a positive PPD generally have an annual CXR; however, this practice is at the discretion of the OHP physician and is often omitted if there are no signs or symptoms of the TB disease in the employee. The findings and recommendations must be fully documented.
3.2.6 Health Maintenance Examinations
3.2.6.1 Introduction
Health Maintenance examinations are voluntary and very effective in maintaining a healthy workforce if incorporated into NASA's overall wellness/health education program. The purpose of NASA's Health Maintenance examination is to maintain a healthy workforce by identifying an individual's potential health risks in order to educate, modify behavior, or refer out for further treatment. The purpose of standardizing these exams is to ensure that all employees have access to the minimum acceptable services that will be the same from Center to Center. An employee transferring from one Center to another will be assured of finding the same basic services and compatible baseline information.
3.2.6.2 Process Description<
a. Federal Employees Health Program (FEHP)
(1) FEHP examinations on civil service personnel are conducted according to the criteria established in this document.
(2) Frequency: Complete physical examinations are given every 3 years, with appropriate initial baseline and interim history. A limited examination may be offered in the interim years.
(3) Complete physical examinations includes the following:
(a) Examination by, or under the auspices of, a physician (including an offer of a total body skin examination, prostate and testicular, or pelvic and breast examinations).
(b) Vital signs - including height, weight, blood pressure, pulse rate and rhythm.
(c) Fasting blood chemistry profile to include glucose and complete lipid profile (total cholesterol, HDL, LDL, triglycerides and total cholesterol/HDL ratio with CBC).
(d) Urinalysis.
(e) Hemocult.
(f) Visual acuity.
(g) 12-lead ECG.
(h) Pap test for females and PSA for males.
(i) Mammograms with periodic followup as described below.
(j) Baseline examinations with followup examinations if clinically indicated (audiogram, chest x-ray, pulmonary functions with Forced Vital Capacity (FVC), Forced Expiratory Volume (FEV) 1, and Forced Expiratory Flow (FEF) 25-75.
(k) Baseline cardiac stress test and/or sigmoidoscopic or colonoscopy (if indicated by history and examination
findings) examination should be offered (within the clinic if NASA standards are met, or from outside resources if unavailable within the clinic), and followup as clinically indicated.
(l) Ocular tonometry for glaucoma (in the clinic if using an air pressure instrument or an outside resource otherwise).
(m) Optional tests offered if clinically indicated may include a thyroid profile including Thyroid Stimulating Hormone (TSH) (with T3 and T4 if TSH abnormal or clinically indicated), skin-fold or BMI.
(4) Limited or partial physicals are offered in the interim years and include an examination and interim history, vital signs, the complete blood chemistry, CBC, urinalysis, hemocult test, or other elements of a screening battery. Females are offered pelvic/pap tests and breast examinations, and males are offered prostate and testicular
examinations annually. Mammograms are offered according to the following
guidelines:
(a) 35 and older - initial baseline.
(b) 40-49 - every 2 years.
(c) 50 and older - annually.
(5) Those who have had breast pathology or breast surgery, including breast implants, should have mammograms at a fixed medical facility rather than with a screening examination in a mobile unit.
(6) Additional tests and/or examinations may be requested at the discretion of the examining official.
(7) Employees, upon written request, may have the results of their examination furnished to their private physician.
(8) Eligibility of other Federal employees (DOD, National Park Service, and Fish and Wildlife Service employees) may be eligible for both the Health Maintenance examination and job-related
occupational physical examinations (eligibility is determined by individual
contracting organizations).
b. NASA Executives
(1) Personnel designated as NASA Executives for physical examinations request their physical examinations in the same manner as for the job-related physical examinations.
(2) Complete and limited physicals for NASA Executives are similar to those described in paragraph a.
3.3 Emergency Services
3.3.1 Life Support Services
3.3.1.1 Introduction
a. Life support services are generally provided as emergency measures. These services are configured to respond to onsite injuries and illnesses, to deal with immediate life threatening conditions, and to stabilize the patient for expeditious transportation to more comprehensive medical services when indicated.
b. Every conceivable situation must be envisioned and a deliberate response designed. Many situations may be definitively managed with resources provided onsite. When circumstances beyond the capabilities of onsite occupational health services are encountered, plans and expedient action must ensue that the patient and additionally required services be brought together.
3.3.1.2. Responsibilities
a. Each NASA Center must plan for and implement at the widest level possible provision to furnish, as the minimal response, Basic Life Support in the timeliest manner feasible. All clinic personnel, including receptionists, and secretaries must have BLS/CPR training. Health professionals must be trained and have medical equipment to provide Advanced (cardiac and/or trauma) Life Support (ACLS) on scene (preplacement when indicated by nature of hazard, as Shuttle launch and landing support) or by rapid response teams (fire and rescue, ambulance teams).
b. Readiness of such capabilities will be assured by
appropriate and current certifications of personnel, by date verification of equipment and medications, and by periodic training exercise of the emplaced capability. To assist maintenance and readiness of equipment and medications, an Emergency Crash Cart Checklist is found in Appendix E.
c. Reserved.
3.3.1.3 Process Description
a. Adequate provision of emergency response must first determine the extent and nature of probable requirements. This is followed with deliberate decisions as to what level and types of response will be furnished onsite, which conditions will be transported to other medical capabilities, and what resources will be required to deliver those responses.
b. Plans, personnel, and equipment must be provided. Plans shall be periodically reviewed for acceptability and currency, personnel will be appropriately trained and certified, equipment and supplies will be maintained and verified functional and current, and training simulations of total response systems will be periodically exercised. Services and their readiness must be documented.
3.3.1.4 References
NPR 8715.2, NASA Emergency Preparedness Plan Procedures and Guidelines.
3.3.1.5 Flow Diagram
The flow diagram for this process is shown in Figure 3.3.1 at the end of this section.
3.3.2 Patient Transportation/Evacuation
3.3.2.1 Introduction
The type of transportation and its frequency of use varies according to the nature of the patient's condition, worksite
location, and NASA Center geography and activities. Evacuation to off site
medical facilities may be by ground or air vehicles as medical situation and distance dictate.
3.3.2.2 Responsibilities
a. At each NASA Center, decisions on type of transportation to be made available are made jointly by medical professionals and management officials. Case use is a medical decision. It is also the Center's responsibility to assure the availability and operational readiness of employed transportation/evacuation services,
including type of en route care provided, when called. All such transport must meet local and State requirements.
b. The NASA OHP assists NASA Centers in their assessment of need for and decisions on providing these services. The OHP PCO receives reports of incidence of transportation/evacuation of patients
from all NASA Centers and evaluates any anomalous events or unusual trends.
The OHP PCO helps to standardize services across Centers to the extent feasible and practicable and periodically audits their status.
3.3.2.3 Process Description
Need for and frequency of patient transportation/evacuation must be assessed by each NASA Center. Method for providing that capability is determined and implemented according to this assessment. Appropriate auditing of quality and readiness of services is conducted by Center officials as well as the OHP. Tracking of Agency statistics and patterns of use for patient transportation/evacuation will be done by the OHP.
3.3.2.4 Flow Diagram
The flow diagram for this process is shown in Figure 3.3.2 at the end of this section.
3.3.3 Automatic External Defibrillator Program
3.3.3.1 Introduction
The OHP supports the use of Automatic External Defibrillators (AED) at NASA Centers in order to provide a timely response to victims of sudden cardiac arrest caused by ventricular fibrillation. Ventricular fibrillation is a treatable condition and potentially survivable when immediate treatment is provided. The goal of this program is to provide a timely emergency response and treatment for sudden cardiac arrest while ensuring the rapid transfer of the individual into the
community EMS. The ability to respond quickly not only increases the potential survival for the individual, it provides the opportunity for the best possible medical outcome.
3.3.3.2 Responsibilities
a. The OHP is responsible for establishing the AED Program Policy and Guidelines and providing support and consultation to the Centers.
b. NASA Center Medical Directors have primary responsibility for implementation and oversight of the AED Program.
3.3.3.3 Process Description
An AED Program must be implemented at all of the NASA
Centers. It provides the needed emergency response critical
, to a good
outcome. Elements of the process include the following:
a. An assessment of Center needs must be completed prior to the planning and implementation stage of the program development.
b. An AED Program requires medical direction and oversight by a physician.
c. The implementation phase includes the development of program policy a
, nd procedures, development of emergency res
pons
, e plan, selection responders, and procurement of equipment and supplies.
d. All responders must receive initial training and certification in CPR and the use of AED's and periodic retraining.
e. The program plan must be integrated into the local community; EMSaston to ensures rapid transfer of care.
f. Documentation is a key component of the program and includes incident responses, drills, training records, and equipment maintenance.
g. An evaluation of each emergency response and emergency drill includes a critique of the response, feedback to the responders, and modification of emergency response plan when indicated.
h. Critical Incident Debriefing is offered to responders after an emergency response when appropriate.
i. Program evaluation is an ongoing process to evaluate the effectiveness of training, emergency responses, and event outcomes.
j. Access to an AED should be within 3 minutes.
3.3.3.4 References
a. Chief Medical Officer's memo "NASA Occupational Health Program Guidelines for Implementing a Center Automatic External Defibrillator Program", July 20, 2000.
b. Public Law 106-505, Public Health Improvement Act, Title IV-Cardiac Arrest Survival, November 13, 2000.
c. 66 FR 28495, Guidelines for Public Access Defibrillation Programs in Federal Facilities May 23, 2001.
3.3.3.5 Flow Diagram
The flow diagram for this process is shown in Figure 3.3.3 at the end of this section.

3.3.4 Treatment Recommendations for Cold Injury Caused by Exposure to Cryogenic Liquids
3.3.4.1 Reserved.
a. Reserved.
b. Reserved.
3.3.4.2 RReserved.
a. Reserved.
b. Reserved.
3.3.4.3 Reserved.
a. Reserved.
b. Reserved.
3.3.4.4 Reserved.
a. Reserved.
b. Reserved.
(1) Reserved.
(2) Reserved.
c. Reserved.
(1) Reserved.
(2) Reserved.
d. Reserved.
(1) Reserved.
(2) Reserved.
(3) Reserved.
3.3.4.5 Reserved.
a. Reserved.
(1) Reserved.
(a) Reserved.
(b) Reserved.
(c) Reserved.
(d) Reserved.
(e) Reserved.
(f) Reserved.
(2) Reserved.
(3) Reserved.
(4) Reserved.
(5) Reserved.
b. Reserved.
(1) Reserved.
(2) Reserved.
(3) Reserved.
(4) Reserved.
(5) Reserved.
(6) Reserved.
c. Reserved.
(1) Reserved.
(2) Reserved.
(3) Reserved.
d. Reserved.s
e. Reserved.
f. Reserved.
g. Reserved.
3.3.4.6. Reserved.
a. Reserved.
b. Reserved.
c. Reserved.
d. Reserved.
3.3.4.7 Reserved.
3.3.5 Medical Response to Nuclear, Biological, and Chemical Warfare Agents
3.3.5.1 Introduction
a. Nuclear, Biological and Chemical (NBC) terrorism incident is an intentional act designed to create fear, maim, and kill the public. NBC incidents have the potential to be extremely dangerous. The importance of this subject cannot be overemphasized, and physicians, nurses, and allied professionals will develop a solid understanding of this subject and the medical armamentarium for dealing with these threats.
b. Various bacteria, fungi, viruses, rickettsial agents and toxins can be used as potential biological warfare agents. Some of the most commonly mentioned ones are Bacillus anthracis (anthrax), and Yersinia pestis (plague) and can be dispersed in aerosols that can remain suspended in the air for hours. Other possible routes of exposure can obviously be contamination of food and water supplies.
c. Chemical warfare agents can consist of various chemical compounds but are broadly divided into 'lethal' agents and 'incapacitating' agents. Some of the commonly mentioned ones are cyanide, mustard gas and nerve agents like acetylcholinesterase inhibitors.
3.3.5.2 Responsibilities
a. The medical management of NBC agents is and must
always be a team effort among medical, safety, environmental, security,
facilities and other relevant disciplines which include Federal, State and
local concerns and regulatory agencies, such as the Nuclear Regulatory
Commission, Department of Defense, Federal Emergency Management Agency, CDC, OSHA, and the Environmental Protection Agency.
b. The NASA's Emergency Preparedness Managers,
designated by each Center Director, are responsible for compliance with all
policies and regulations applicable to their Centers and for developing plans and resources to meet the needs from an NBC incident.
c. NASA Center OHP professionals, including EAP, must develop and implement requirements and strategies for dealing with an NBC
incident, including promoting awareness and maintaining anticipatory readiness, and critical incident debriefing. They also must assure liaisons with Center safety and Emergency Preparedness, and security personnel, as well as other relevant disciplines. This is especially essential with the local police, medical, and public health communities.
d. The NASA OHP provides oversight and guidance to
Centers and coordinates intercenter and interagency allocation of resources in incidents involving mass casualties.
3.3.5.3 Process Description
a. NASA Centers develop a coordinated, detailed and clear NBC response plan and conduct response exercises and training regularly.
b. This plan should include but not be limited to the
following:
(1) Potential target sites/ target personnel.
(2) Resources needed and their management.
(3) Initial response.
(4) Early hazard identification.
(5) Handling of mass causalities/fatalities.
(6) Evacuation, containment, transportation.
(7) Triage and treatment.
(8) Decontamination, personal protective equipment.
(9) Organizations and personnel.
(10) Additional responses.
(11) Command control, communications, alerts and
notifications.
(12) Handling chaos, mass hysteria.
(13) Crime scene and evidence preservation.
(14) Consider State and Federal support, how to prepare for their arrival, and how to maximally utilize their resources.
3.3.5.4 References
a. Medical Management of Biological Casualties
Handbook, U. S. Army Medical Research Institute of Infectious Diseases, Fort Detrick, Maryland.
b. Medical Management of Chemical Casualties Handbook, U.S. Army Medical Research Institute of Chemical Defense, Aberdeen Proving Ground, Maryland.
3.3.5.5 Flow Diagram
The flow diagram for this process is shown in Figure 3.3.5 at the end of this section.
3.4 Infection Control Program
3.4.1 Infection Control
3.4.1.1 Introduction
a. Infection control is an organizationwide program that requires the involvement and commitment of all health care personnel and employees. The program should be a systematic, coordinated, and continuous approach to improving performance, focusing on surveillance, prevention, and control of infections. The scope of the function is broad; it includes activities at the direct patient care level and at the patient care support level to reduce risks of nosocomial/clinic-acquired
infections in patients. Activities are also designed to reduce risks of
transmission of infections among civil service personnel, contractors, health care personnel, students, and visitors. Particular areas of interest for infection control are direct patient care practices, ancillary services such as laboratory, radiology, and rehabilitation, and support services such as linen supply.
b. The goal of the infection control program is to identify and reduce the risks of endemic and epidemic nosocomial/clinic-acquired infections in patients and health care workers. Infection control practices must encompass infections that patients may acquire as a result of their care or treatment within the occupational health facility as well as protect health care providers in these settings. To facilitate implementation and assessment of this task, the PCO has developed a checklist that Occupational Health personnel may use to assess their program. The checklist is shown in Appendix F.
3.4.1.2 Responsibilities
a. Center Chief Medical Officers/Medical Directors will ensure that an infection control program is established and maintained at their Centers. These officials are responsible for ensuring that adequate resources, including time and training, are available to support the program.
b. The infection control program must be the responsibility of at least one person designated by the Center Chief Medical Officer/Medical Director. That individual is known as the Infection Control Officer (ICO) and is responsible for overseeing the program. Specific knowledge and training relevant to infection control will enable the designated person to keep up to date on regulatory changes.
3.4.1.3 Process Description
a. The Infection Control Plan plays an integral role in ensuring that the occupational health clinic's infection control program
operates efficiently. The designated ICO establishes, maintains and oversees this process. It is recommended that the ICO establish an Infection Control Committee (ICC). The ICC membership should consist of a physician, a nurse, and any other staff necessary to manage the program effectively. The ICC should coordinate all activities related to the surveillance, prevention, and control of nosocomial infections.
b. It is the duty and responsibility of the ICO and/or ICC to develop, implement, and maintain an infection control plan and guidelines that meet the needs of the occupational health facility. The plan should include program goals, surveillance activities, infection control guidelines, infection control training, nosocomial/clinic-acquired infections reporting process, program assessment, performance improvement procedures and program documentation. After the initial plan is developed, it is reviewed every 2 years based on the proceeding year's infection control data by the ICO/ICC. The review should include infectious waste disposal, shelf life of all stored sterile items, reprocessing of nondisposable items, housekeeping contract, linen services, radiology, and laboratory services.
c. The infection control guidelines should provide an easy reference to important infection control guidelines and practices. The infection control guidelines and practices address patient care issues such as hand-washing practices, approved antiseptics and disinfectants, sterilization of equipment and disinfecting the clinic, laundry, housekeeping, ventilation, and environmental sampling. There must be a health program for the health care personnel, including immunizations, postexposure protocols and work restrictions/accommodations. The Center Bloodborne Exposure Control Plan and a tuberculosis prevention and control plan are also included as part of the guidelines and practices. The infection control guidelines and practices must be reviewed and updated every 2 years by the ICO/ICC.
d. Infection control issues and data, including infections and communicable diseases, immunization status of health care personnel and tuberculosis skin testing conversion data, will be reviewed and summarized on a regular basis by the ICO or ICC to determine if trends are being formed. Appropriate action must be taken on all infection control issues or problems and a process for followup established to ensure effectiveness of the corrective action. To ensure compliance with infection control standards, the ICO and/or the ICC must conduct facility inspections at least annually.
e. The ICO must ensure that all health care personnel and facilities comply with applicable Federal, State, and local regulations
including notification of the public health agency when patients or health care personnel are treated for infectious or communicable disease.
f. The training of health care personnel is either required by Federal (OSHA) regulations or strongly recommended. The following infection control training is required:
(1) Newly assigned health care personnel must receive infection control training within 10 days of placement in clinical environment.
(2) Health care personnel must receive infection control training, including OSHA Bloodborne Pathogen, universal precautions and Personal Protective Equipment (PPE), annually.
(3) Health care personnel must receive training when
significant regulatory changes occur.
(4) Health care personnel providing direct care to patients should receive continuing education on patient care practices to minimize the risk of nosocomial-acquired infections.
g. Personnel should have copies of training materials, general, and infection control reference materials available to them. All training and continuing education records must be kept in the health care personnel records for a minimum of 3 years.
3.4.1.4 References
a. Occupational Safety and Health Administration (OSHA) Regulations.
b. The Centers for Disease Control (CDC) Isolation Precautions and Infection Control in Hospital Personnel.
c. Healthcare Infection Control Practices Advisory
Committee (HICPAC).
d. Joint Commission on Accreditation of Healthcare
Organizations (JCAHO) Standards.
3.4.1.5 Flow Diagram
The flow diagram for this process is shown in Figure 3.4.1 at the end of this section.
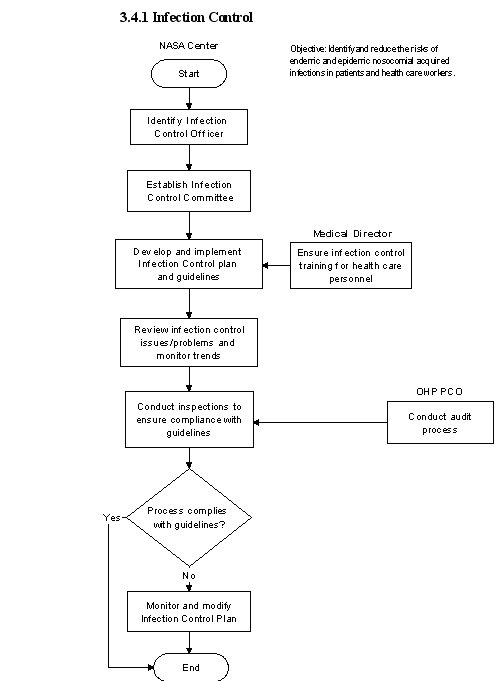
3.4.2 Universal Precautions
3.4.2.1 Introduction
a. The objective of Universal Precautions is to prevent parenteral, mucous membrane, and skin exposure of health care workers to bloodborne pathogens and potentially infectious materials. Universal precautions are designed to decrease the risk of transmission of bloodborne pathogens. These precautions are intended to supplement rather than replace recommendations for routine infection control, such as using gloves and hand washing. Universal precautions assume that all blood and body fluids must be treated as infectious, regardless of knowledge of the patient's status. Since the risk of transmission from feces, nasal secretions, sputum, sweat, tears, urine, and vomitus is extremely low or nonexistent, precautions do not apply to these fluids unless they contain visible blood.
b. Infection control requires the employer and employee to assume that all human blood and specified human body fluids are infectious for hepatitis B, hepatitis C, HIV, and other bloodborne pathogens. The CDC guidelines on universal precautions should be consistently used for all patients and situations in which job exposure to blood or potentially infectious materials may occur and, especially in emergency situations, in which the risk of exposure is increased.
3.4.2.2 Responsibilities
a. The Medical Director and Chief Nurse are responsible for ensuring that infection control procedures are followed.
b. The NASA Center Medical Director is responsible for ensuring training within the first 10 days of employment for individuals at risk for occupational exposure and annually thereafter.
c. Any person who, in the performance of duties, could reasonably be expected to come into contact with blood or other potentially infectious materials is responsible for being successfully trained according to the CDC guidelines and OSHA Standards, Part 1910.1030. It is important for employees who are exposed to blood or body fluids to become familiar with their rights and the employer's obligations.
d. The NASA OHP PCO reviews compliance with infection control and universal precaution practices.
3.4.2.3 Process Description
Healthcare workers can minimize exposure of their skin or mucous membranes to potentially infective materials with the use of protective barriers. Appropriately designated personal protective barriers such as gloves, gowns, masks, and protective eyewear will be used for all tasks and procedures in which occupational exposure to blood or body fluids is anticipated. If health care workers have exudative lesions or weeping dermatitis, they should refrain from all direct patient care and from handling patient care equipment until the condition resolves. The risk of transmission of bloodborne pathogens can be minimized if health care
workers use the CDC recommendations for universal precautions.
3.4.2.4 References
a. OSHA Standard - 29 CFR Part 1910.1030, Occupational Exposure to Bloodborne Pathogens.
b. Publication (CPL 2-2.44C) Enforcement Procedures for the Occupational Exposure to Bloodborne Pathogens Standard.
c. CDC Morbidity and Mortality Weekly Report, Recommendations for Prevention of HIV Transmission in Healthcare Settings, 1987; 36 (Supplement No. 2S).
d. CDC Morbidity and Mortality Weekly Report, Perspectives in Disease Prevention and Health Promotion Update: Universal Precautions for Prevention of Transmission of Human Immunodeficiency Virus, Hepatitis B Virus, and Other Bloodborne Pathogens in Healthcare Settings, 1988; 37(24); 377-388.
3.4.2.5 Flow Diagram
The flow diagram for this process is shown in Figure 3.4.2 at the end of this section.
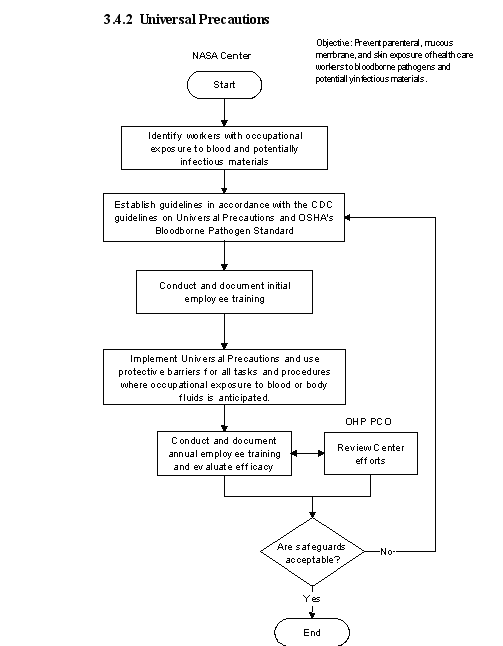
3.4.3 Bloodborne Pathogens
3.4.3.1 Introduction
a. On March 6, 1992, the OSHA Bloodborne Pathogen Standard, 29 CFR 1910.1030, took effect. Bloodborne pathogens are pathogenic microorganisms that are present in human blood and can cause disease in humans. Hepatitis B Virus (HBV), Hepatitis C Virus (HCV), and HIV are three of the most serious diseases caused by these pathogens.
b. The standard covers any person, including janitorial workers, who can reasonably expect to come in contact with blood or potentially infectious materials as part of their job. Infection control requires the employer and employee to assume that all human blood and specified human body fluids are infectious for HIV, HBV, and other bloodborne pathogens. Where differentiation of body fluids is difficult, all body fluids are to be considered as potentially infectious.
c. "Good Samaritan" acts such as assisting a coworker who has a nosebleed would not be covered.
3.4.3.2 Responsibilities
a. The Center Medical Director is responsible for
establishing procedures and treatment availability for employees exposed to bloodborne pathogens and for providing training and hepatitis B vaccine to employees with potential occupational exposure. The health care opinion must comply with OSHA regulations.
b. NASA Centers are responsible for establishing a written Bloodborne Pathogen (BBP) Exposure Control Plan that identifies workers with occupational exposure to blood and other potentially infectious material.
c. NASA Center OHP personnel are responsible for
maintaining confidentiality of testing.
d. The NASA OHP periodically reviews Center programs.
3.4.3.3 Process Description
a. The BBP Exposure Control Plan must specify a means to protect and train the employees. Through the Exposure Control Plan, on-the-job risks for all employees exposed to blood and body fluids will be reduced.
(1) The plan must be accessible to employees and updated annually or when new or revised procedures are implemented.
(2) The methods of compliance include universal precautions, engineering and work practice controls, personal protective equipment, housekeeping, and handling of laundry.
(3) The standard also covers Hepatitis B vaccination, postexposure evaluation and followup, hazard communication requiring biohazard warning labels, employee training, and recordkeeping.
b. NASA Centers must provide any person, who in the
performance of their duties could reasonably be expected to come in contact
with blood or other potentially infectious materials, training according to
OSHA standards and offer the Hepatitis B vaccination within the first 10 days of employment at no cost.
c. A declination form must be signed if the employee
chooses not to be vaccinated for hepatitis B. If the employee later chooses to receive the vaccine, the employee will receive it at no cost. Universal precautions MUST be observed. It is important for employees who are exposed to blood or body fluids to become familiar with their rights and the employer's obligations.
3.4.3.4 References
a. OSHA Standards - 29 Part CFR 1910.1030 Occupational Exposure to Bloodborne Pathogens.
b. Publication (CPL 2-2.44C) Enforcement Procedures for the Occupational Exposure to Bloodborne Pathogens Standard.
c. NASA SOLAR Learning and Resources (online Web site at
http://solar.msfc.nasa.gov/solar/delivery/disc/its/private/cgi-bin/certific)
3.4.3.5 Flow Diagram
The flow diagram for this process is shown in Figure 3.4.3 at the end of this section.

3.4.4 Immunizations
3.4.4.1 Introduction
Many employees are at risk for exposure to and possible transmission of vaccine-preventable diseases because of their work environment or contact with co-workers, patients, or infective material. Maintaining immunity is a vital part of prevention and infection control for workers. The objective is to utilize immunizations to reduce and protect employees from becoming infected through exposure and potential transmission of diseases to other workers. A number of immunizations may be indicated or considered, depending on the risk of exposure to the employee.
3.4.4.2 Responsibilities
The NASA Center OHP is responsible for providing immunizations for international travel and health maintenance. The Centers will have educational materials on adult immunizations available for employees.
3.4.4.3 Process Description
a. All employees should be evaluated for conditions
related to communicable diseases at the time of employment and at periodic
health maintenance exams. This should include medical history, immunization status, and assessment for conditions that may predispose personnel to acquiring or transmitting communicable diseases. It is strongly advised that employees working in the health care field be immunized against hepatitis B, measles, mumps, rubella, influenza, diphtheria, tetanus, and varicella according to the CDC and the Advisory Committee on Immunization Practices (ACIP). At this time, the CDC does not recommend annual tuberculosis testing of health care workers in facilities that are at minimal or very low risk for tuberculosis exposure.
b. Since international travel has become more common for employment, employees should be evaluated and educated in advance of travel regarding health risks. Protective immunization guidelines are in the Health Information for International Travel published by the CDC. The CDC also provides a telephone consultation service and FAX-back service. Evaluation and/or testing should be performed as necessary, especially if illness occurred during or after travel.
c. An immunization record should be maintained for each employee and reviewed periodically. The record should reflect documented disease and vaccination histories as well as immunizing agents administered during employment. At each immunization encounter, the record should be updated, and the employee encouraged to maintain the record as appropriate. Tetanus and diphtheria status should be reviewed and given, if appropriate, for all employees injured at work. In special circumstances, such as working with lab animals or research, rabies and polio may be suggested. Other considerations important to employees include work restrictions for susceptible workers who are exposed to vaccine-preventable diseases and control of outbreaks in the workplace setting. Exposed workers should be evaluated regarding the circumstances surrounding the exposure, symptoms, and the need for postexposure prophylaxis and treatment. Postexposure work restrictions ranging from limited duty to complete exclusion from duty are appropriate for workers who are not immune to certain vaccine-preventable diseases.
3.4.4.4 References
a. 1994 CDC Guidelines for Preventing the Transmission of Mycobacterium Tuberculosis in Healthcare Facilities.
b. Centers for Disease Control (CDC) Morbidity and Mortality Weekly Report, Immunization of Healthcare Workers: Recommendation of the Advisory Committee on Immunization Practices (ACIP) and the Hospital Infection Control Practices Advisory Committee (HI
, CPAC), December 26, 1997, Vol. 46, No. RR-18.
c. American College of Occupational and Environmental Medicine (ACOEM) Guidelines for Employee Health Services in Healthcare Facilities, V.1.0, 1998.
d. Infection Control and Hospital Epidemiology, Vol. 20, No. 10, October 1999.
3.4.4.5 Flow Diagram:
The flow diagram for this process i
s shown in Fi
, gure 3.4.4 at the end of this section.
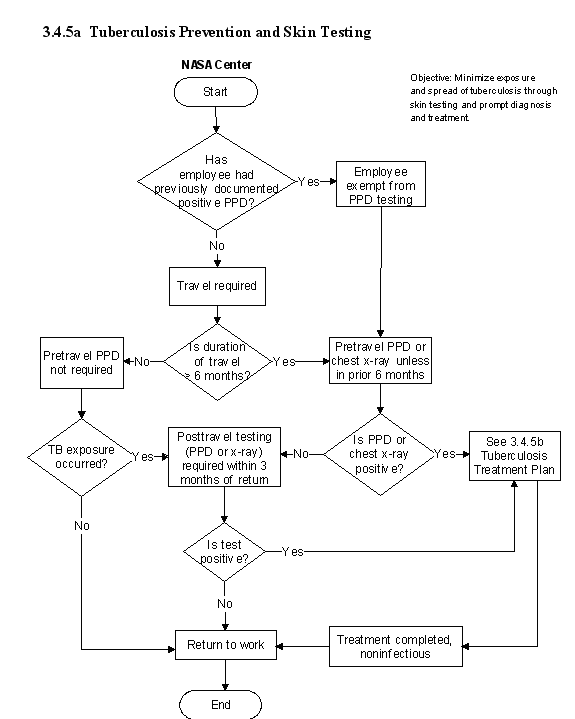
3.4.5 Tuberculosis Prevention and Skin Testing
3.4.5.1 Introduction
NASA employees are at risk for exposure to tuberculosis from international travel or assignment. Every NASA Center should offer traveler health information as an integral part of the Center's international travel services. The goal is to minimize and monitor exposure to tuberculosis by providing Mantoux/Purified Protein Derivative (PPD) skin testing for tuberculosis to all travelers as part of a prevention program.
3.4.5.2 Responsibilities
a. Each NASA Center Medical Director shall assure that the Center OHP services offer traveler tuberculosis testing to NASA and contractor employees if specified in their contract traveling on official NASA business. Travelers to Russia, newly independent states, and to developing countries may be especially susceptible to tuberculosis exposure.
b. The NASA OHP PCO is responsible for providing current procedures and guidelines to NASA Headquarters and NASA Centers for
safeguarding the health of their employees during international travel and
assignment. This responsibility includes monitoring of a tuberculosis control program.
3.4.5.3 Process Description
a. NASA recommends and encourages a Mantoux/PPD test for tuberculosis periodically for travelers.
b. Pretravel testing is recommended if the duration of travel is less than 6 months unless the employee was previously tested in the past six months. Pretravel testing is required if travel is over 6 months in duration. Employees with a previously recorded positive skin test are exempt from testing.
c. Posttravel testing is recommended within 3 months of return if the duration of travel is less than 6 months and required if travel is over 6 months in duration. Employees with a previously recorded positive skin test are exempt from testing, but a chest x-ray will be recommended if travel was over 6 months in duration. A chest x-ray will be required if a skin test converts from a previously negative reading to a positive reading. A chest x-ray showing active disease will require medical treatment and removal from work until a medical clearance from the employee's treating physician indicates that the employee is no longer infectious. Testing of close family members and work associates may be recommended.
d. An employee with a skin test conversion (a previously documented negative test which becomes positive on retesting) with a negative (no indication of infection) chest x-ray should be considered for prophylactic treatment with medication. An employee with a positive skin test (without a previously documented negative test) is considered a reactor. An employee who is a reactor should have a chest x-ray, be treated as a converter if the x-ray is positive (indicates signs of active disease), and start treatment for the disease. The employee will not need prophylactic treatment with medication if the x-ray is negative.
3.4.5.4 Flow Diagram
The flow diagram for this process is shown in Figure 3.4.5a and 3.4.5b at the end of this section.

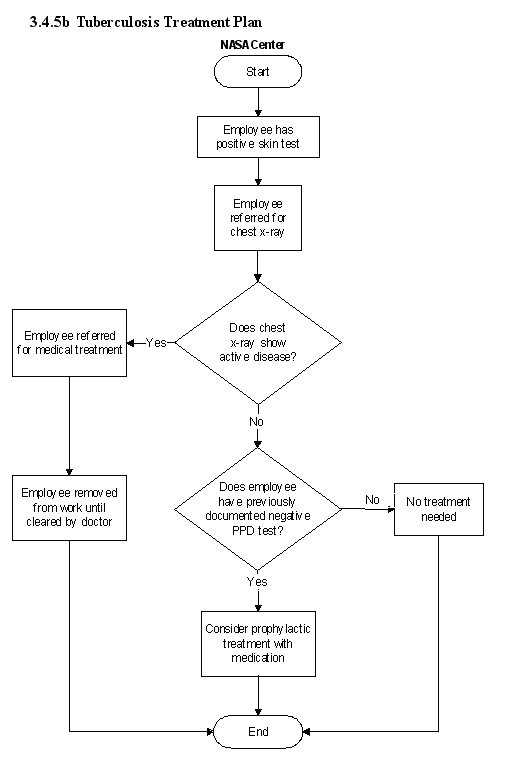
DISTRIBUTION:
NODIS
This Document is Obsolete and Is No Longer Used.
Check the NODIS Library to access the current version:
http://nodis3.gsfc.nasa.gov
![[NASA Logo]](../Images/nasaball.gif)















